This article has been
cited by other articles in ScienceCentral.
PRESENTATION OF CASE
Dr. Hee Sun Cho: A 62-year-old woman was hospitalized for jaundice and general weakness of 1-week duration. The patient was taking medications for paroxysmal atrial fibrillation, hypertension, hyperlipidemia, and gastroesophageal reflux disease. Atrial fibrillation was diagnosed six months prior to her admission, and she was prescribed amiodarone 400 mg, aspirin 100 mg, and ilaprazole 10 mg. She had taken pelubiprofen, acetaminophen, mosapride, rebamipide, and melatonin for 3 weeks prior to admission. In her family's medical history, her father had lung cancer, and her mother had liver cancer. She denied alcohol consumption, smoking, or a history of using herbal medication. She had no history of elevated liver enzyme levels or liver diseases.
She also had fatigue, poor oral intake associated with nausea and anorexia, and weight loss of 6 kg over 2 months. Her vital signs were stable: blood pressure was 127/71 mmHg, heart rate was 63 beats per minute, respiratory rate was 20 beats per minute, and body temperature was 37.0°C. Icteric sclerae were observed with no signs of abdominal tenderness, hepatomegaly, or splenomegaly. Her heartbeat was regular, and she had clear lung sounds on auscultation. She weighed 51 kg and was 163 cm in height, and her body mass index was 19.1 kg/m2.
The initial laboratory findings were as follows. A complete blood count showed no significant findings. The blood chemistry results showed elevated aspartate transaminase (AST) (276 U/L), alanine transaminase (ALT) (412 U/L), alkaline phosphatase (ALP) (140 U/L), γ-glutamyl transpeptidase (γ-GTP) (483 U/L), total bilirubin (12.61 mg/dL), direct bilirubin (9.38 mg/dL), along with albumin (3.6 g/dL) and ammonia (59 mcg/dL). The international normalized ratio was slightly prolonged (1.21). Amylase, lipase, and cholesterol levels were within normal range. Laboratory findings also showed elevated iron (346 mcg/dL) and ferritin (995.6 ng/mL) levels with a transferrin saturation of 96%.
For the diagnosis, further serological examinations were performed. The patient tested negative for anti-mitochondrial antibody. Furthermore, hepatitis B surface antigen, IgM antibodies against the hepatitis A virus, IgM antibody to the hepatitis B core antigen, and the anti-hepatitis C virus antibody were not detected. IgM antibody for cytomegalovirus and Epstein-Barr virus antigens were negative. Antineutrophil cytoplasmic antibodies (ANCA), anti-smooth muscle antibody (SMA), and anti-liver kidney microsomal antibody (anti LKM-1 Ab) were negative, although anti-nuclear antibody (ANA) was positive (1:200, speckled). Total immunoglobulin G levels were within the normal range.
IMAGING OBSERVATIONS
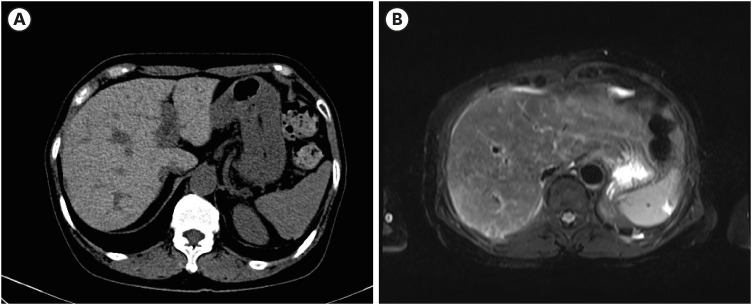
Dr. Ji Hoon Kim: As the patient was jaundiced and had a cholestatic pattern of liver enzyme elevation, liver ultrasonography was performed. On liver sonography, heterogeneous and coarse liver parenchyma was observed, with increased vascular echogenicity, suggesting acute hepatopathy. No bile duct dilatation, gallbladder abnormalities or portal vein thrombosis were observed. Abdominal computed tomography (CT) showed a high density of liver parenchyma, which was markedly higher than that in the spleen, especially in the non-contrast-enhanced scan, with additional findings of diffuse gallbladder wall thickening without gallbladder stones and the absence of common bile duct dilatation (
Fig. 1A). Liver magnetic resonance imaging (MRI) showed diffuse low signal intensity in the liver on T2 weighted images and no specific findings in the gallbladder and bile duct (
Fig. 1B). These findings suggest that there is no abnormality in the biliary trees. Further serological examination was required to investigate the cause of the abnormal liver test results.
Fig. 1
Imaging findings at the hospitalization. (A) Non-contrast phase imaging of computed tomography showing high density of the liver (B) T2-weighted imaging of magnetic resonance imaging showing the low signal intensity of the liver.
CLINICAL IMPRESSION
Dr. Hee Sun Cho: Drug-induced liver injury (DILI), autoimmune hepatitis (AIH), secondary hemochromatosis could be considered as the diagnosis.
DIFFERENTIAL DIAGNOSIS
Dr. Hee Sun Cho: Regarding the positive ANA results, AIH could have led to jaundice and abnormal liver function test results. However, ANCA, SMA, and anti-LKM tests were negative, and immunoglobulin G levels were within normal range. According to the simplified criteria for the diagnosis of AIH,
1 our laboratory findings and the absence of viral hepatitis were insufficient to diagnose AIH. A liver biopsy and typical pathological findings of AIH would be required to confirm a diagnosis of AIH.
2
Laboratory findings showed elevated iron (346 mcg/dL) and ferritin (995.6 ng/mL) levels with a transferrin saturation of 96%. A high density of liver parenchyma on pre-enhanced abdomen CT scan and low signal intensity on liver MRI could suggest a diagnosis of hemochromatosis. However, imaging results do not have a high sensitivity for diagnosing this disease. Primary hemochromatosis is extremely rare in Korea,
3 although we did not perform genetic testing to confirm it. As the patient had no history of iron overload or physical signs suggestive of this, secondary hemochromatosis is of less concern. In patients with inflammation and hepatocellular necrosis, serum ferritin levels may be elevated in proportion to body iron stores owing to the increased release from tissues,
4 although ferritin has various pathological meanings which are yet to be completely understood.
5 Therefore, it is advisable to repeat serum ferritin levels after the acute hepatocellular damage has subsided. In this case, transferrin saturation had decreased to fall within the normal range after 2 weeks of conservative care, dropping from 96% to 35%.
DILI is diagnosed, excluding other causes of liver disease, by assessing the temporal association between the use of drugs and abnormal liver tests. Most drugs are associated with liver injury within 6 months of initiation and all the drugs the patient had used were prescribed within 6 months of their admission.
6 Possible alternative causes, such as hemochromatosis or AIH, should be excluded, but they were not strongly suspected, although pathologic confirmation was needed. LiverTox provides information regarding the incidence, clinical manifestations, diagnosis, and management of DILI for each medication.
7 The current guidelines recommend searching for suspicious medications that may cause DILI.
6 Among the medications, amiodarone was highly suspected of causing liver injury with a likelihood score of A. To assess the relationship between amiodarone and acute hepatic injury, the modified Roussel Uclaf Causality Assessment Method (RUCAM) scale was used, which is commonly used to quantitatively assess causality in cases of suspected DILI.
8 Finally, it is critical to assess the improvement in liver tests upon drug withdrawal.
6
PATHOLOGICAL FINDINGS
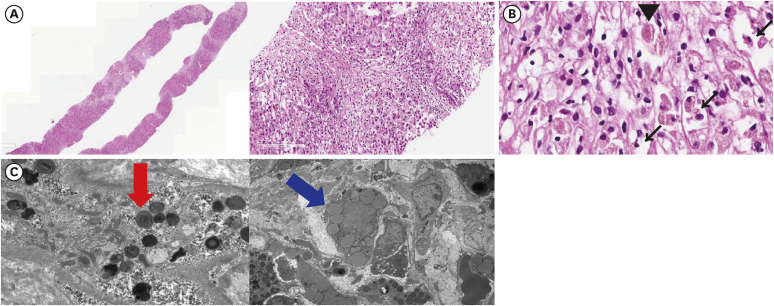
Dr. Ji Won Han: A liver biopsy was performed to confirm the diagnosis. Hematoxylin and eosin (H&E) staining showed severe lobular and mild porto-periportal activity with no fibrosis, suggesting acute liver injury (
Fig. 2A, left). Furthermore, there was marked centrilobular necrosis of the hepatocytes, with the presence of ballooned hepatocytes and Mallory bodies (
Fig. 2A, right). In the dyeing hepatocytes, unspecified eosinophilic granules were observed (
Fig. 2B). To further characterize these granules, an electron microscope was used. The examination showed enlarged lysosome with myelin-like figures, which had a densely packed, concentrically arranged structure, suggesting phospholipid-laden lysosomal lamellar bodies (
Fig. 2C, left). Fat granules were also deposited in the cytoplasm (
Fig. 2C, right). These findings are consistent with the acute liver injury caused by amiodarone.
Fig. 2
Histologic findings obtained from the initial liver biopsy. For the confirmation of diagnosis, liver biopsy was performed. (A-C) Histological findings of the initial liver biopsy. Hematoxylin and eosin stain was done. ×20 (left) and ×100 (right) slides showing severe lobular, portal, and periportal inflammation with hepatocyte necrosis (A). ×400 slide shows a red granule (arrowhead) among the necrotic cells. Ballooned hepatocytes and Mallory bodies are seen. (black narrow arrows) (B) Electron microscopy shows phospholipid laden lysosomal lamellar bodies (left, red arrow) and fatty deposition in the lysosome (right, blue arrow) (C).
CLINICAL COURSE
Dr. Hee Sun Cho: Our patient underwent conservative treatment, including hydration and hepatotonics, and the discontinuation of amiodarone, which was thought to be the most probable cause of the acute hepatitis. As Holter monitoring confirmed sinus conversion of atrial fibrillation, the cessation of amiodarone was maintained under the consultation of cardiologists. Another antiarrhythmic agent was not commenced until a later review in the outpatient clinic. After a week of administration, her liver chemistry tests had decreased by more than 50% without the aid of additional treatment including steroid therapy. The liver chemistry results were AST (92 U/L), ALT (120 U/L), and total bilirubin (5.51 mg/dL). Levels of bilirubin, AST, ALT, ALP, and γ-GTP were within the normal range at 3 months post-diagnosis (
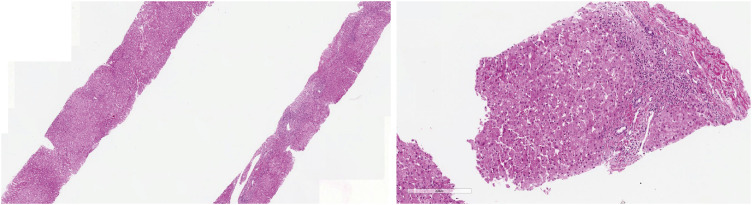
Fig. 3). A follow-up liver biopsy was performed 3 months after the initial liver biopsy, to confirm the improvement of necrosis and inflammation without fibrosis (
Fig. 4). The modified RUCAM score was 9 points, indicating that amiodarone was a highly probable cause of acute liver injury in this case.
Fig. 3
Serial laboratory findings from admission to 5 months after cessation of amiodarone. Serum levels of total bilirubin, AST, ALT, and γ-GTP at admission and 1 week, 1 month, 3 months, 5 months after the cessation of amiodarone are presented.
γ-GTP = γ-glutamyl transpeptidase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, Bx = biopsy, D/C = discharge, F/U = follow up.
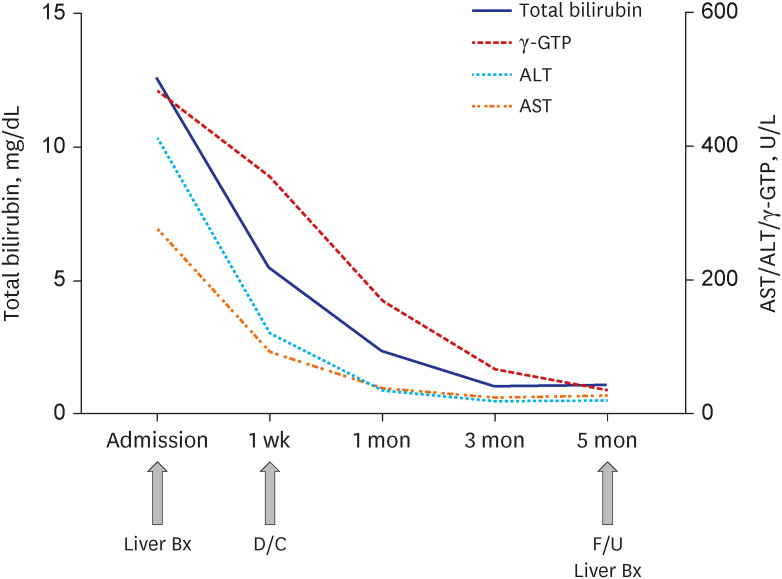
Fig. 4
Histologic findings obtained from liver biopsy 5 month after amiodarone withdrawal. H&E stain was done. ×20 (left) and ×100 (right) slides H&E staining from the liver biopsy samples at 5 months after the cessation of amiodarone showing significant recovery from the liver injury.
H&E = hematoxylin and eosin.
FINAL DIAGNOSIS
Dr. Pil Soo Sung: DILI, caused by amiodarone.
GENERAL INTRODUCTION OF THE DISEASE MANAGEMENT
Dr. Heechul Nam: Cessation of the causative drug is the most important management strategy of DILI.
6 Some drug ingestions can be therapeutically managed, such as N-acetylcysteine for paracetamol overdose and L-carnitine for valproic acid overdose. In some clinics, hepatotonics with antioxidizing properties may be used.
9 Corticosteroid therapy for DILI has a limited role and is reserved for patients with immune-mediated DILI or ‘drug-induced’ AIH.
6 With the withdrawal of the causative drug, liver injury improves without any additional treatment, which confirms a diagnosis of DILI.
PROGNOSIS
Dr. Pil Soo Sung: Amiodarone has a large apparent volume of distribution, and even after discontinuation, the total body stores do not decline for several weeks.
10 DILI caused by amiodarone is most commonly observed as a sole elevation in aminotransferases without liver dynfunction.
11 However, in rare cases, severe liver injury or fulminant hepatitis may also occur.
12 Amiodarone-induced DILI can also progress to cirrhosis, resulting in decompensated hepatic failure,
13 although this rarely occurs.
14
DISCUSSION
Dr. Pil Soo Sung: DILI is a common cause of liver injury. It includes the entire spectrum, from asymptomatic elevation in liver tests to acute liver failure. Drugs that produce predictable (high incidence) liver injury, such as paracetamol, usually do so within a few days and are generally the result of direct liver toxicity of the parent drug or its metabolites. Unpredictable (idiosyncratic, low prevalence) events manifest as overt or symptomatic disease and can occur with intermediate (1–8 weeks) or long (1 year) periods of latency.
6 Symptoms can suggest acute hepatitis, cholestasis, or a mixed presentation.
6 As there are no diagnostic tests or biomarkers for idiosyncratic DILI, its diagnosis is made after the stringent exclusion of other causes of liver disease, assessing the temporal association between the use of drugs and abnormal liver tests, looking for the characteristic features of the drug, and assessing improvement in liver injury upon drug withdrawal. Tools for assessing DILI, such as RUCAM, may be helpful.
6 A liver biopsy can help exclude other causes of liver disease and identify specific features associated with the use of different drugs.
6 As in our patient, a thorough medical history and characteristic histopathological findings helped in diagnosing this patient with DILI due to amiodarone. Initially, our patient presented with marked centrilobular necrosis of hepatocytes, but 3 months after cessation of the drugs, a dramatic improvement was observed histopathologically and this improvement was represented with the blood chemistry. Overall, this case is a representative case of an ideally diagnosed and treated DILI due to amiodarone.
Dr. Si Hyun Bae: Histological findings of amiodarone-induced DILI are characterized by steatosis, inflammation, fibrosis, and phospholipidosis.
15 The histologic features of amiodarone-induced liver injury are similar to those of alcoholic hepatitis, as they are also characterized by steatosis, leukocyte infiltration, and hepatocyte ballooning. The presence of phospholipidosis by electron microscopy is the morphological hallmark of amiodarone-induced liver injury. As with our patient, phospholipidosis is identified by phospholipid-loaded lysosomal lamellar bodies. Practical guidelines for amiodarone treatment demonstrate that plasma concentration of amiodarone poorly correlated with its toxicity.
16 As a result, pharmacokinetic testing has a very limited role in predicting or diagnosing amiodarone hepatotoxicity. However, CT scans may be useful in detecting amiodarone-induced liver injury. As the iodine content of amiodarone is deposited in the liver tissue, the characteristic high-density liver parenchyma can be observed through CT scans. When amiodarone-taking patients show elevated liver enzymes, amiodarone-induced liver injury should be suspected if a characteristic high-density liver is observed on CT scans.
ACKNOWLEDGMENTS
The Case Conference section is prepared from monthly case conference of Department of Internal Medicine, the Catholic University of Korea College of Medicine, Seoul, Korea.
References
1. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008; 48(1):169–176. PMID:
18537184.

2. Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol. 2021; 27(1):58–69. PMID:
33291862.

3. Lee JY, Yoo KH, Hahn SH. HFE gene mutation, C282Y causing hereditary hemochromatosis in Caucasian is extremely rare in Korean population. J Korean Med Sci. 2000; 15(2):179–182. PMID:
10803694.

4. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011; 54(1):328–343. PMID:
21452290.

5. Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, et al. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun. 2022; 126:102778. PMID:
34883281.
6. Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023; 77(3):1036–1065. PMID:
35899384.
7. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases;2012.
8. Low EX, Zheng Q, Chan E, Lim SG. Drug induced liver injury: East versus West - a systematic review and meta-analysis. Clin Mol Hepatol. 2020; 26(2):142–154. PMID:
31816676.

9. Lee HA, Chang Y, Sung PS, Yoon EL, Lee HW, Yoo JJ, et al. Therapeutic mechanisms and beneficial effects of non-antidiabetic drugs in chronic liver diseases. Clin Mol Hepatol. 2022; 28(3):425–472. PMID:
35850495.

10. Buggey J, Kappus M, Lagoo AS, Brady CW. Amiodarone-induced liver injury and cirrhosis. ACG Case Rep J. 2015; 2(2):116–118. PMID:
26157932.

11. Gregory SA, Webster JB, Chapman GD. Acute hepatitis induced by parenteral amiodarone. Am J Med. 2002; 113(3):254–255. PMID:
12208392.

12. Gayam V, Khalid M, Dahal S, Garlapati P, Gill A, Alex R, et al. Fatal acute liver failure with intravenous amiodarone: a case report and literature review. Gastroenterol Res. 2018; 11(1):62–63.

13. Jaiswal P, Attar BM, Yap JE, Devani K, Jaiswal R, Wang Y, et al. Acute liver failure with amiodarone infusion: a case report and systematic review. J Clin Pharm Ther. 2018; 43(1):129–133. PMID:
28714083.
14. Sung PS, Yoon SK. Amiodarone hepatotoxicity. Hepatology. 2012; 55(1):325–326. PMID:
21898482.

15. Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009; 62(6):481–492. PMID:
19474352.

16. Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med. 2000; 160(12):1741–1748. PMID:
10871966.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download