INTRODUCTION
South Korea’s coronavirus disease 2019 (COVID-19) vaccination program was implemented in February 2021. BNT162b2 (Pfizer Biotech) and ChAdOx1-nCoV-19 (Oxford/AstraZeneca) were started at first, followed by mRNA-1273 (Modena) and JNJ-78436735 (Janssen) in the second quarter of 2021. With the nationwide schedule for vaccination, our medical center started immunizing with BNT162b2 and ChAdOx1 in March 2021, and to evaluate vaccine effectiveness in healthy healthcare workers (HCWs), a study analyzing neutralizing antibodies was conducted.
1 As a result, both vaccines (BNT162b2 and ChAdOx1) showed a 100% antibody production rate after the primary vaccination, but ChAdOx1 showed a significant decrease in the protective immune response compared to BNT162b2 with more than 68% cutoff of surrogate virus neutralization test (sVNT) inhibition.
According to reports from the Korea Disease Control and Prevention Agency (KDCA), in December 2021, the first vaccine coverage rate was 83.7% of the total population in South Korea, and the second was 81.2%. However, variants of the alpha (B.1.1.7), beta (B.1.351), gamma (P.1.), and delta (B.1.617.2) were reported to reduce the effectiveness of the existing vaccine. The omicron (B.1.1.529), the most recent and dominant variant that appeared in November 2021, was reported to neutralize the vaccine effect. The existing variants and the continuous emergence of new variants have increased the need for an extra booster dose.
23
Six months after the second dose vaccination, all medical staff at the center were given a booster dose with the BNT162b2. Existing studies have reported the side effects of cross-inoculation by a booster dose, and its effectiveness in prevention has yet to be determined. There are also questions as to how effective the current vaccine would be against variants.
The current study aimed to analyze the levels of neutralizing antibodies after booster vaccination in response to the wild type, delta, and omicron variants. We also examined the changes in immunogenicity against the omicron variants after COVID-19 infection, due to the rapid spread of omicron infections.
METHODS
Study design and participants
This study was designed as a follow-up study to a previously published cross-sectional cohort study.
1 One hundred and five HCWs who had received the BNT162b2 booster vaccine and expressed voluntary participation were enrolled in the current study (
Fig. 1). All participants had no history of COVID-19 infection or suspected symptoms at the time of registration.
Fig. 1
Flowchart of the COVID-19 vaccine study in HCW cohorts at Soonchunhyang University Bucheon Hospital.
COVID-19 = coronavirus disease 2019, HCW = healthcare worker, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
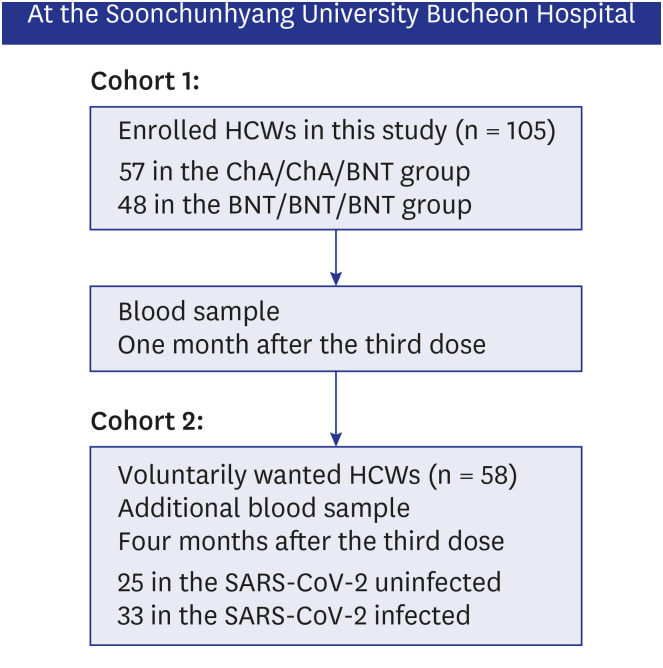
Blood samples of participants in cohort 1 were collected at four weeks after the booster dose. The samples were analyzed with a commercial virus neutralization test kit (Genscript Biotech Corporation, Piscataway, NJ, USA), which was used in the previous study.
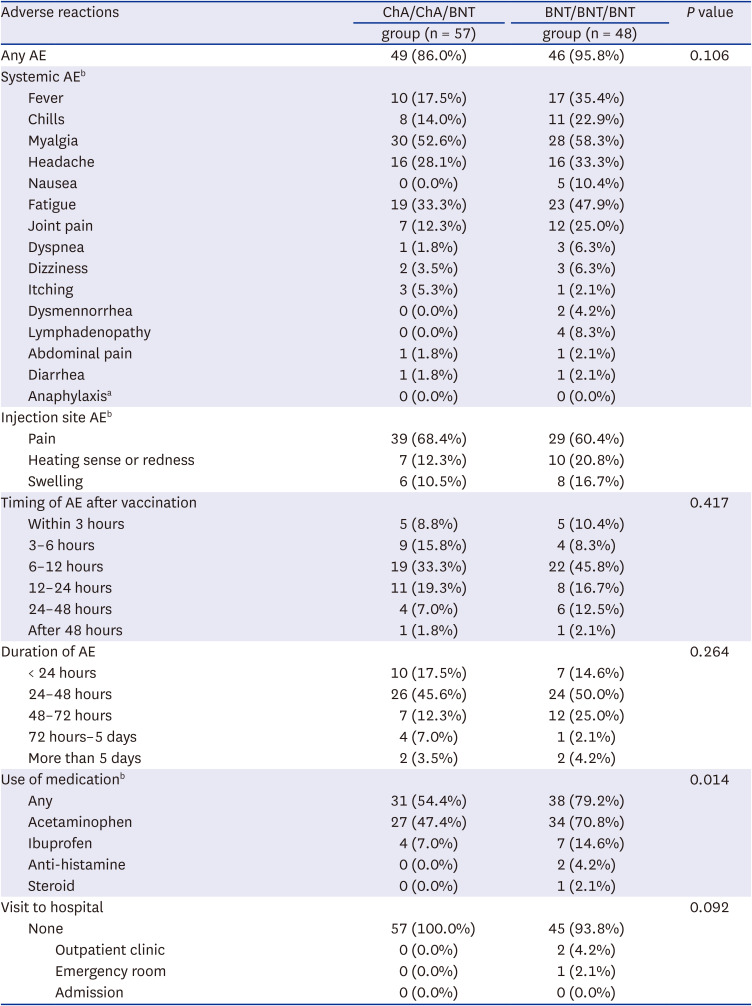
1 Participants who consented to the study were given a self-administered questionnaire for adverse events of the booster vaccination. The questionnaire included the following information: gender, age, date of vaccination, previous history of COVID-19 infection, drug AEs, allergy, type and duration of AEs, use of medication, and need for medical attention (visit to outpatient clinic or emergency room).
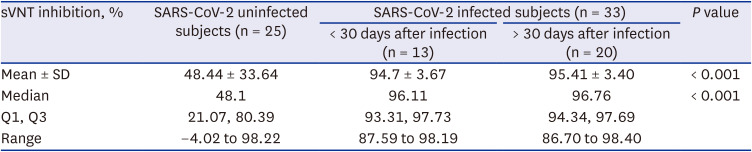
During the study period, the omicron variant rapidly spread in South Korea and several HCWs in cohort 1 were confirmed with COVID-19. Therefore, additional blood samples were collected to compare the change in neutralizing antibody inhibition scores after omicron infection, and to assess the difference between omicron-infected and uninfected participants in cohort 2. The blood sampling was limited to HCWs who voluntarily consented at least three months after the booster dose.
For the control group, we used samples stored in the biobank of Soonchunhyang University Bucheon Hospital, which is a member of the Korea BioBank Network. The control group was comprised of healthy adults with no COVID-19 vaccinations and no confirmed COVID-19 infection.
Cohorts and groups
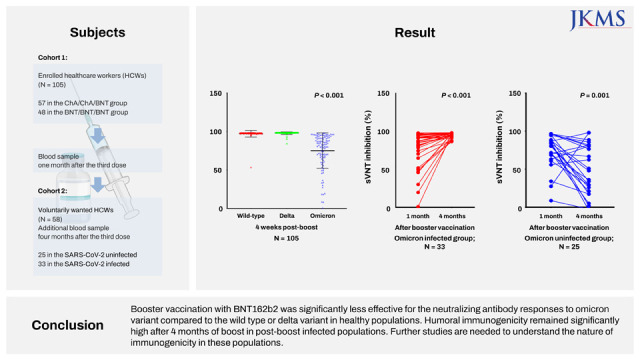
Cohort 1 evaluated the neutralizing antibody inhibition score against the wild type, delta, and omicron variants one month after the booster vaccine, and cohort 2 was a group for further study of the change in neutralizing antibody inhibition score against the omicron variant four months after the booster dose.
We defined primary vaccination as two doses (first and second dose) of BNT162b2 vaccine (Pfizer) or two doses of ChAdOx1 vaccine (AstraZeneca/Oxford), and the third dose as the booster vaccination. The BNT/BNT/BNT group was administered a booster dose with BNT162b2 (Pfizer) after two doses of the BNT162b2 (Pfizer) vaccine, three weeks apart. The ChA/ChA/BNT group was administered a booster dose with BNT162b2 (Pfizer) after two doses of the ChAdOx1 vaccine (AstraZeneca/Oxford), 12 weeks apart.
Serological assays
The ELISA-based sVNT was used to evaluate neutralizing activity against the wild-type SARS-CoV-2 and the variants of B.1.617.2 (delta) and B.1.1.529 (omicron). All detailed methods were described in the previous study.
1
Statistical analysis
Statistical analyses were performed with GraphPad Prism software (version 9.3.1) and R software (version 4.0.2). All measurements and calculation data are presented as the mean ± standard deviation (SD), median, interquartile range, range for continuous variables, and frequency (percentage) for categorical variables. The nonparametric Kruskal-Wallis test was used to compare two or more independent groups for the continuous variables. Independent two-sample t-tests or χ2 tests were used for comparison of the independent group variables. The paired t-test was used for the one-subject variable. The repeated measure analysis of variance test was used to compare two or more dependent groups for the continuous variables. All tests were two-tailed, with P < 0.05 considered statistically significant.
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Soonchunhyang University Bucheon Hospital (IRB No. 2021-12-017). Written consent was obtained from all enrolled participants.
DISCUSSION
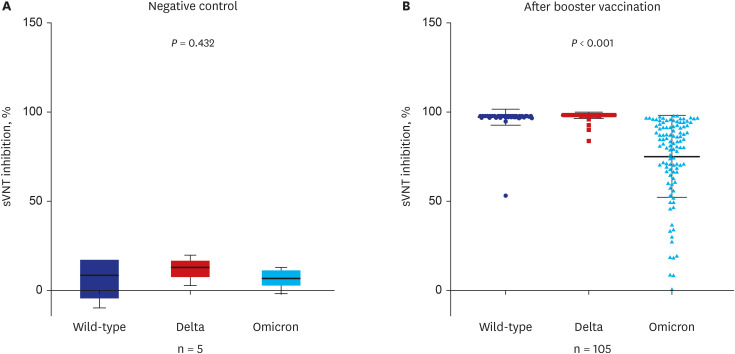
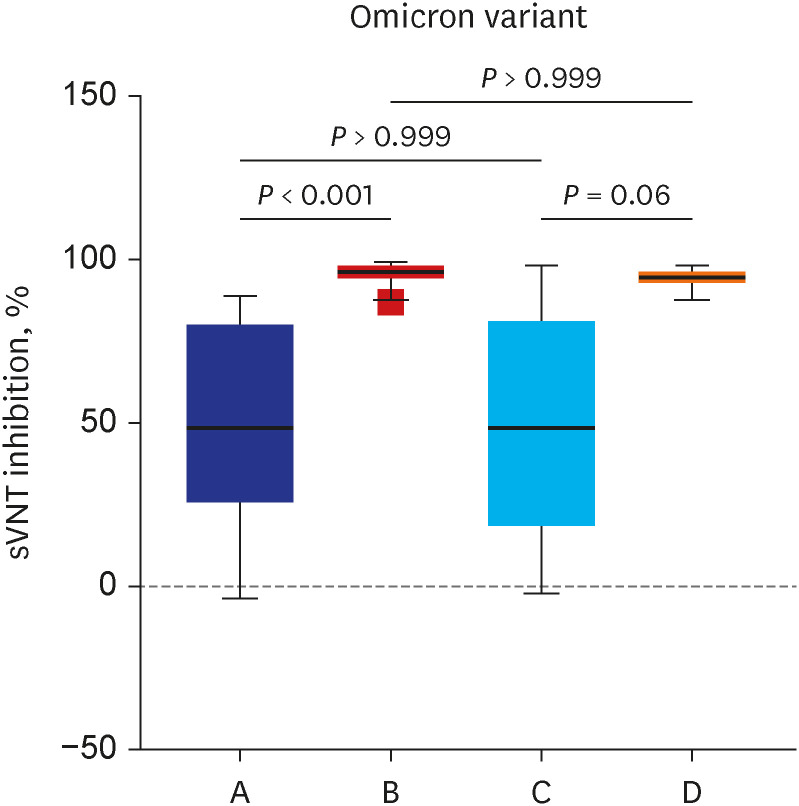
The current study showed that the neutralizing activity against the omicron variant after a booster dose was significantly lower than the wild type or delta variants (75% vs. 98%,
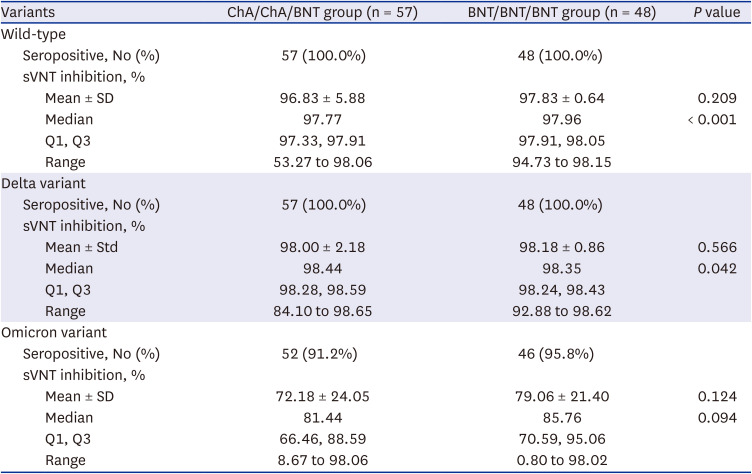
P < 0.001). There was no difference in reduced humoral immunogenicity against the omicron variant between the ChA/ChA/BNT group and the BNT/BNT/BNT group. Our results are supported by a recent study showing that the neutralizing activity for omicron was 6 to 23 times lower than delta with the BNT162b2 (Pfizer) booster.
4 In December 2021, the UK Health Security Agency (UKHSA) announced the delta variant prevention rates in the ChA/ChA/BNT group as 94% and the BNT/BNT/BNT group as 93%, but the omicron variant showed rates of 71% in the ChA/ChA/BNT group and 76% in the BNT/BNT/BNT group.
5 Although the current study for sVNT inhibition values used a surrogate antibody, the delta variant prevention rates were 98% for the ChA/ChA/BNT group and the 98% for the BNT/BNT/BNT group and the omicron variant prevention rates were 72% for the ChA/ChA/BNT group and 79% for the BNT/BNT/BNT group.
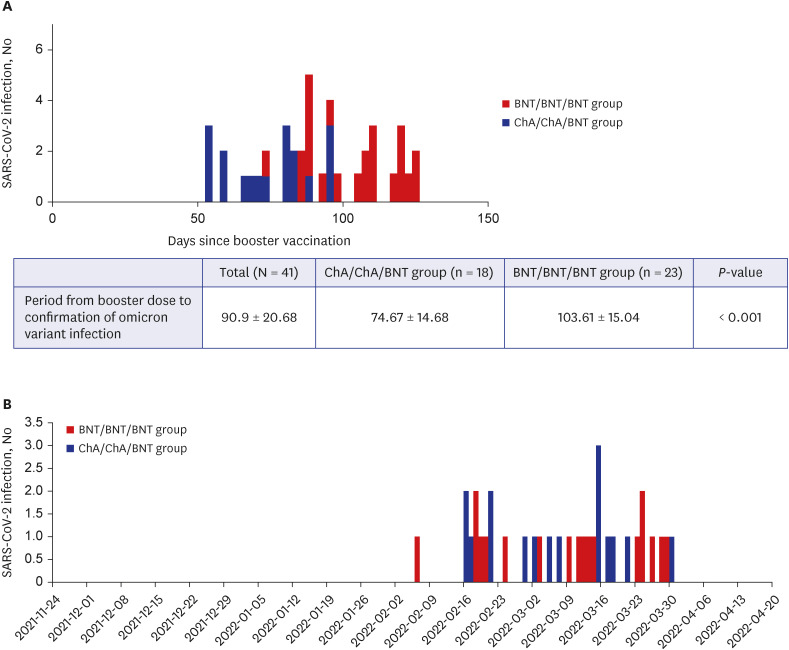
While observing that both homogeneous and heterogeneous boosters have low sVNT levels against the omicron variant (mean sVNT 72% in the ChA/ChA/BNT group vs. 79% in the BNT/BNT/BNT group), interestingly, among the 41 participants with omicron infections, those with a heterogenous booster were infected in a shorted period of time than those with a homogenous booster (75 vs. 104 days,
P < 0.001). Such a result may imply that early vaccine effectiveness against the omicron variant in the ChA/ChA/BNT group was lower than that in the BNT/BNT/BNT group. However, the time difference between homogeneous boosters and heterogeneous boosters seems unclear. Thus, the reduced effectiveness of the booster vaccine is probably related to increased omicron transmissibility rather than enhanced immunologic escape after the booster vaccination, as suggested by Yu et al.
6
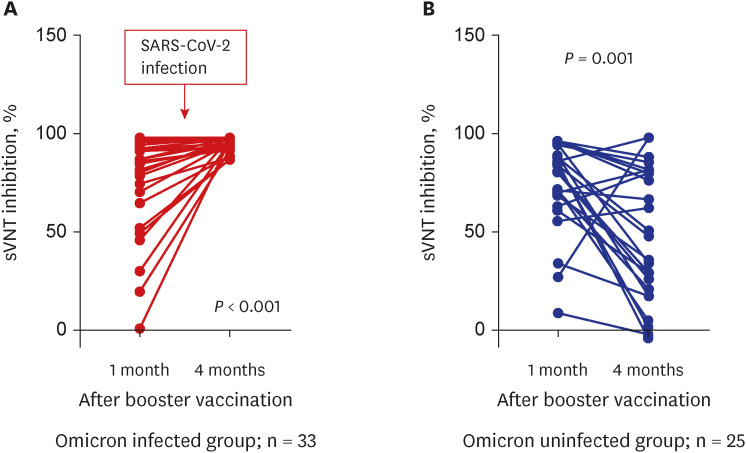
This is the first study to examine the change in neutralizing antibody inhibition scores after a breakthrough omicron infection after the third vaccination (booster dose). In addition, we compared the change in neutralizing antibody inhibition scores in the uninfected group in the cohort corresponding to the same conditions. There was a significant decrease in the sVNT inhibition level four months after the third booster vaccine, however, a dramatic increase was observed after a breakthrough infection. Of course, additional research is needed to determine the meaning of the sVNT inhibition level raised after infection. We are conducting further studies to determine the duration of antibody maintenance and the ability to prevent infection in this subset of populations.
According to the announcement by the KDCA on July 19, 2022, 2.8% of confirmed COVID-19 patients were reinfected, and 97% were first infections.
7 Our results support the evidence for a COVID-19 resurgence. The inhibition level of neutralizing antibodies against omicron in the breakthrough omicron-infected group (95%) was almost identical to wild type (97%) or delta variants (98%) after the third booster vaccination. Of course, there are variables such as cross-immune reactions, but additional research is required to determine whether there will be a uniform or increased neutralizing antibody inhibition score after a fourth vaccination (booster dose) using the original vaccine in the noninfected group.
This study used a sVNT, which showed a correlation with the gold standard for cell culture-based neutralization assays.
18910 Delta and omicron variants were evaluated in the same way using the sVNT. Although there are few studies using sVNT for novel variants,
11 a recent study showed that the neutralizing antibody inhibition score against the delta variant, which was measured by sVNT, strongly correlated with pseudotyped virus neutralization tests.
12 As mentioned above, the inhibition score of neutralizing antibody in our study was consistent with the effectiveness of real vaccines reported by the UKHSA. It can be seen that the sVNT method is helpful in reflecting actual vaccine effectiveness against delta and omicron variants.
Although this study has the advantage of using real-world data on a group of HCWs in South Korea, it has some limitations. First, due to the characteristics of the study subjects including a relatively larger number of young women, the results of this study may not be generalized to other populations. The follow-up periods of the ChA/ChA/BNT and BNT/BNT/BNT groups were also different because homologous and heterogeneous booster vaccines were not equally available, resulting in a time difference of one month. In addition, we could not confirm whether the COVID-19 infected HCWs had omicron variants due to difficulties in analyzing the whole genome sequencing of infected cases in our hospital. However, the COVID-19 infection of our subjects mainly occurred for a month from the end of February 2022, and according to a press release from the KDCA on April 12, 2022, the detection rate of omicron in the third week of February 2022 was 98.9%, and the first week of April was 100%,
13 therefore, we could infer that most of the HCWs infected at the time were infected with the omicron variant. Finally, cellular immune responses that may affect cross-immune responses have not been evaluated.
Recently, the rapid transmission of BA.2, BA.4, and BA.5 in omicron sublineages with additional mutations has been reported. There are also controversies over the pros and cons of the fourth additional vaccination using the original vaccine. The additional vaccination may be helpful to those with weakened immunity or due to disease or age. However, recommendations for booster vaccination in populations that became infected after booster vaccination are still foggy.
14
In conclusion, booster vaccination with BNT162b2 is less effective in terms of humoral immunogenicity against the omicron variant compared to wild-type or delta variants. However, booster vaccines are still recommended in the absence of a variant-specific vaccine, as they are significantly effective in preventing severe diseases, especially in a high-risk population. In addition, research is needed to identify the immunological characteristics of a group that does not belong to the high-risk population and has hybrid immunity through COVID-19 infection after booster vaccination.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download