Infectious complications are the leading cause of morbidity and mortality after liver transplantation (LT) in our region, especially hospital-borne infections [1,2]. Furthermore, a lack of medical insurance adds a tremendous financial burden when these patients’ stay is prolonged for the management of multiple complications. Enhanced recovery after surgery (ERAS) has enabled fast-track LT at many centers around the world [3-5]. We, therefore, changed our transplant protocols for selected patients and applied a super-fast-track pathway (preoperation to discharge). Our patients were discharged at 5.5±1.6 days after LT on average. All are alive, are receiving regular follow-up, and have not experienced any complications until the time of writing this report. We therefore briefly present our super-fast-track discharge protocol. Nine blood group-compatible liver transplants were done between October 2021 and August 2022. Among them, three were living-donor LT and six were deceased-donor LT. Patients’ preoperative and perioperative demographics are shown in Table 1 and Fig. 1.
Preoperative: our main aim was to optimize all patients’ conditions prior to LT. In short, they were given hepatoprotective medication and intravenous (IV) albumin (20%, 100 mL; Baxter) once a week and followed up once a week with blood biochemistry reports, and the medication was adjusted accordingly. Decompensation was managed quickly and the aim was to keep the patients infection-free during the preoperative period. Nutritional build-up was done as much as possible (high protein [60 g/day], no salt, and fluid restricted to <1.5 L per day).
Intraoperative: the anesthesia protocol was similar to that described in a previous study [3]. Surgery was performed according to the standard protocol. The total blood transfusion was minimized (1.8±0.8 units). The bench time was kept short (30.1±7.7 minutes). Induction was done with an IV injection of Solu-Medrol (500 mg; Pfizer). All nine patients were extubated on the table and then transferred to the liver intensive care unit (LICU) with continuous monitoring.
Postoperative: one critical care physician and a 1:1 nursing-to-patient ratio was maintained until the patient was discharged from the LICU. Early ambulation and oral intake were started 12 hours later. Liver Doppler ultrasonography and blood biochemistry were done twice a day until postoperative day (POD) 2. Central and arterial lines and the Foley catheter were removed on POD 2, and thereafter noninvasive monitoring was done until discharge. Immunosuppression (calcineurin inhibitors+mycophenolate mofetil) was started early, as soon as the lactate level was <2 mmol/L. IV Solu-Medrol was tapered and converted to oral prednisolone from POD 4. IV antibiotics were given until POD 3, until the trough level was adjusted to 8–10 ng/mL, and then converted to oral. Education for the patient and family on medication, hygiene, nutrition, and wound care was started on POD 1, and the patient was discharged with a home care nurse. Follow-up was done after 3 days with reports, and the medication was adjusted if needed. Then, patients were followed up once a week until 1 month, twice a week until 3 months, and thereafter at 3-month intervals until 1 year. In conclusion, herein we report our brief protocol for super-fast-track discharge of selected LT patients.
REFERENCES
1. Varghese J, Gomathy N, Rajashekhar P, Venugopal K, Olithselvan A, Vivekanandan S, et al. 2012; Perioperative bacterial infections in deceased donor and living donor liver transplant recipients. J Clin Exp Hepatol. 2:35–41. DOI: 10.1016/S0973-6883(12)60081-4. PMID: 25755404.
2. Khillan V, Kale P, Pamecha V, Rathor N, Sarin SK. 2017; Infections in live donor liver transplant recipients: a study of timeline, aetiology and antimicrobial resistance of bacterial and fungal infections from the developing world. Indian J Med Microbiol. 35:604–6. DOI: 10.4103/ijmm.IJMM_17_295. PMID: 29405159.
3. Rodríguez-Laiz GP, Melgar-Requena P, Alcázar-López CF, Franco-Campello M, Villodre-Tudela C, Pascual-Bartolomé S, et al. 2021; Fast-track liver transplantation: six-year prospective cohort study with an enhanced recovery after surgery (ERAS) protocol. World J Surg. 45:1262–71. DOI: 10.1007/s00268-021-05963-2. PMID: 33620540. PMCID: PMC8026463.
4. Sellers D, Sapisochin G, Selzner N, McCluskey SA. 2022; Enhanced recovery for liver transplantation: a first step on a long road. Transplantation. 106:460–1. DOI: 10.1097/TP.0000000000003809. PMID: 33966026.

5. Pollok JM, Tinguely P, Berenguer M, Niemann CU, Raptis DA, Spiro M, et al. 2023; Enhanced recovery for liver transplantation: recommendations from the 2022 International Liver Transplantation Society consensus conference. Lancet Gastroenterol Hepatol. 8:81–94. DOI: 10.1016/S2468-1253(22)00268-0. PMID: 36495912.
Fig. 1
(A) Trends in total bilirubin, (B) serum glutamic pyruvic transaminase (SGPT), and (C) serum glutamic-oxaloacetic transaminase (SGOT) in all nine patients at postoperative day (POD) 0, POD 3, and at the time of discharge.
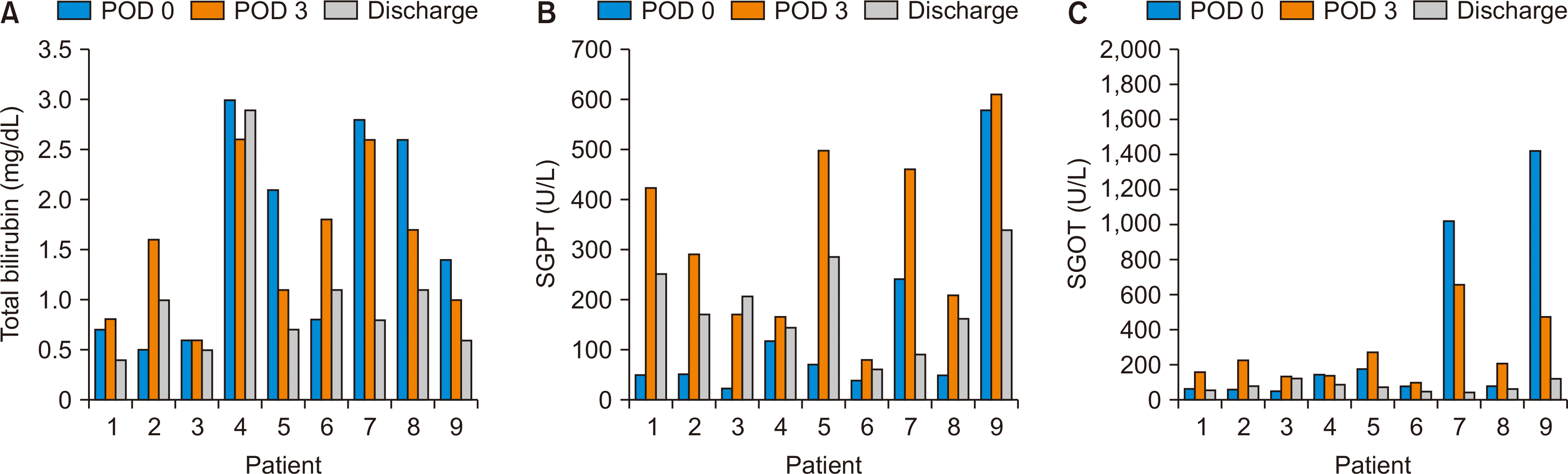
Table 1
Background demographic, preoperative, and postoperative characteristics of all recipients
SD, standard deviation; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; DDLT, deceased donor liver transplantation; LDLT, living-donor LT; TIPS, transjugular intrahepatic portosystemic shunt; UGI, upper gastrointestinal; HRS, hepatorenal syndrome; HE, hepatic encephalopathy; MELD, model for end stage liver disease; CTP, Child-Turcotte-Pugh score; CIT, cold ischemic time; WIT, warm ischemic time.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download