Abstract
Adrenal and spinal metastases of hepatocellular carcinoma (HCC) are rare entities with significant morbidity and mortality, particularly after liver transplantation (LT). We report a case of a 49-year-old man who underwent LT for hepatitis B-related end-stage liver disease and HCC (single 4.5 cm lesion [T1N0], without vascular invasion) in 2016. Eighteen months later, adrenal metastasis and hepatitis B seropositive conversion were developed with normal serum tumor. Adrenal metastasis was treated with radiation therapy (RT) and hepatitis B showed spontaneous seronegative conversion. However, 35 months later, spinal metastasis occurred with elevation of the protein induced by vitamin K absence or antagonist-II (PIVKA-II) level (197 mAU/mL), along with hepatitis B seropositive conversion. After sorafenib, sequential regorafenib with RT led to partial response of the spinal lesions, along with hepatitis B seronegative conversion and normal PIVKA-II levels. After 9 months of regorafenib combined with RT, two recurrent lesions were found, as well as hepatitis B seropositive conversion and lesions were treated with transarterial chemoembolization. The patient survived for more than 71 months after LT and 53 months after recurrence under various combinations of therapy. Combined systemic and locoregional therapies can be a treatment option for HCC recurrence, even in LT patients.
Adrenal metastasis of hepatocellular carcinoma (HCC) is rare and can be treated with resection, local ablative therapy, or systemic therapy [1]. According to the Barcelona Clinic Liver Cancer strategy, in advanced stages with extrahepatic metastases, systemic therapy is the first-line treatment option, with an expected survival of more than 2 years [2].
Bone metastasis due to HCC is also rare, with a reported incidence of 3%–20%, and has a very poor prognosis. Moreover, spinal metastases in HCC are even rarer, with frequencies ranging from 0.2% to 2.2%, and are associated with significant morbidity and mortality [3].
Post-liver transplantation (LT) HCC recurrence rates range from 8% to 20% according to a risk group analysis [4]. This recurrence rate has an impact on lower overall survival rates than those observed in non-HCC recipients. Furthermore, post-LT HCC recurrence patterns are different from those in non-transplant settings; as more than 50% are extrahepatic multiple metastases, and disease progression is faster in post-LT settings than in non-transplant settings.
Recent progress in patient selection, intensive surveillance protocols, and perioperative management has improved the survival of HCC recipients. Aggressive management involving locoregional therapy combined with chemotherapy for post-LT HCC recurrence has improved patients’ survival outcomes. Unfavorable factors prognostic factors for survival after HCC recurrence include poorly differentiated original tumors, major vascular invasion observed in explant pathology, unresectable disease, and bone metastases [5]. Prior to 2010, the 3-year survival rate after HCC recurrence following LT treatment was 16%, but the 5-year survival rate subsequently improved to 15%–20% [6].
However, there is no definite treatment for post-LT HCC recurrence. Sorafenib is the most frequently applied treatment, used in 37% of patients, followed by a combination of systemic and locoregional therapies in 18% of patients. Approximately one-third of affected patients receive palliative best supportive care [4].
Herein, we report the case of a patient who was successfully treated for sequential adrenal, spinal, and graft HCC metastases after LT with combined systemic and radiation therapy.
This study was approved by the Institutional Review Board (IRB) at Seoul National University Hospital (IRB No. 2208-001-1345). Informed consent was waived by the IRB due to the retrospective study design.
This report describes the case of a 49-year-old man with acute-on-chronic hepatitis B (HB) and alcoholic-related liver cirrhosis with HCC. His medical Model for End-Stage Liver Disease score without the additional HCC was 37, indicating the need for continuous renal replacement therapy due to encephalopathy, hepatic renal syndrome, and ascites. Variceal bleeding was controlled using balloon-occluded retrograde transvenous obliteration. Deceased donor LT was performed while the patient was admitted to the intensive care unit. The HCC was a treatment-naive, 4.7 cm, single mass (within the Milan criteria) without extrahepatic metastasis or vascular invasion (Fig. 1). The patient’s pretransplant alpha-fetoprotein (AFP) level was 9.3 ng/mL, and the protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 1,614 mAU/mL. Explant pathology revealed a 4.7-cm single, Edmondson-Steiner grade 2 without vascular invasion. The patient was HB treatment-naive. The pretransplant HB surface antigen (HBsAg) titer was 1,587 IU/mL; the HB e antigen (HBeAg) was negative; anti-HBe was positive, and the HB viral DNA titer was 3,590 IU/mL. The brain-dead donor was 60 years old without a history of HB virus (HBV) infection, and had negative HBsAg, positive anti-HBs, and positive HBV core antibody immunoglobulin G. The donor’s serum HB DNA level was not measured. The patient’s recovery was uneventful, and he was discharged on postoperative day 18.
Quadruple immunosuppression therapy consisted of basiliximab, tacrolimus, steroids, and mycophenolate mofetil. Steroids were discontinued 4 months after transplantation. Dual therapy with tacrolimus and mycophenolate mofetil was administered for 8 months as a maintenance therapy (Fig. 2). The patient was switched to tacrolimus monotherapy because a sufficient tacrolimus trough level was achieved. HB prophylaxis was achieved using combination therapy with HB immunoglobulin (HBIG) and entecavir.
The HCC surveillance protocol in the outpatient clinic used tumor markers, and imaging studies were performed every 4 months for 2 years. After transplantation, both AFP and PIVKA-II levels were within the normal range, and HB seronegative conversion was achieved.
Right adrenal metastasis was observed on computed tomography (CT) scans (Fig. 3A), and mild uptake was seen on 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) CT scans (Fig. 3B) 18 months after transplantation. At that time, the AFP level was 2.0 ng/mL and the PIVKA-II level was 22 mAU/mL. Seropositive conversion of HBsAg and HB DNA occurred without graft dysfunction under combination therapy with HBIG and entecavir. Radiotherapy (RT; 48 Gy for four fractions) was performed for the adrenal lesion; a mammalian target of rapamycin inhibitor (mTORi) was added, and the tacrolimus level was reduced. Following the addition of RT and mTORi therapy, the size of the adrenal HCC lesion was reduced and seronegative conversion status was achieved without changing the combination therapy of HBIG and entecavir.
Unfortunately, a follow-up CT scan (Fig. 3C and D) performed 5 months after the initial treatment for metastasis and 23 months after transplantation revealed that the right adrenal lesion had regrown. At this time, the AFP level was 3.7 ng/mL, and the PIVKA-II level was 37 mAU/mL. The HBsAg and DNA were negative with combination therapy. Secondary RT (60 Gy in eight fractions) was performed on the adrenal lesion. Subsequently, the adrenal HCC lesion was reduced in size, and the patient was stable.
One year after the second recurrence, the PIVKA-II level increased to 197 mAU/mL, and HB seropositive conversion reappeared under combination therapy with HBIG and entecavir. CT revealed bone metastasis at the fourth lumbar pedicle (Fig. 4A). Sorafenib was administered at a dose of 400 mg twice daily for 7 months. Despite sorafenib treatment, the PIVKA-II level continued to rise to 4,210 mAU/mL, and the bone metastasis lesion was progressive according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [7] (Fig. 4B). We switched from sorafenib to regorafenib (160 mg/day) combined with RT as a tertiary treatment (32 Gy in four fractions). Subsequently, PIVKA-II levels decreased to 45 mAU/mL, and HBsAg converted to negative. The adrenal (Fig. 4C) and spinal lesions (Fig. 4D) were classified as stable disease according to the RECIST criteria [7].
Nine months after the third recurrence, conversion to HB seropositivity reappeared. At this time, the AFP level was 2.6 ng/mL, and the PIVKA-II level was 41 mAU/mL. Thus, we performed follow-up CT scans and magnetic resonance imaging, which showed five metastatic nodules, at segment 3, 4, and 7 (Fig. 5), although the metastases on the adrenal gland and vertebral pedicle were stable. For those liver metastases, transarterial chemoembolization was conducted (a mixture of 4 mL of Lipiodol and 20 mg of adriamycin with gelfoam). The regorafenib treatment was discontinued. The patient survived for 71 months after transplantation, 53 months after HCC adrenal recurrence, and 17 months after HCC bone recurrence.
Risk factors for HCC-specific mortality include biomarkers such as high AFP or PIVKA-II levels, 18-FDG PET scan positivity, and tumor number and size [8]. Jamtani et al. [9] reported that the 5-year HCC-specific mortality and recurrence rates in low-risk patients were 2% and 5%, respectively; those in middle-risk patients were 12% and 14%, respectively, and those in high-risk patients were 71% and 82%, respectively. In this particular patient, although the pretransplant HCC was within the Milan criteria, the PIVKA-II level was high. The patient was in critical condition shortly before transplantation, and 18-FDG PET scans were not obtained.
In this case, when the first and second adrenal metastases were found on the surveillance imaging studies, both AFP and PIVKA-II levels were in the normal ranges. For this reason, imaging surveillance of posttransplant HCC recurrence is important. In addition, the PIVKA-II level, instead of the AFP level, increased at the time of bone and graft recurrence. PIVKA-II levels in HCC patients represent a more aggressive tumor nature, as well as metastatic lesions. Thus, follow-up of both AFP and PIVKA-II levels as tumor markers is important, in addition to imaging studies.
A previous study showed that second-line regorafenib after sorafenib treatment increased the overall survival of recipients after transplantation. Locoregional treatment for HCC recurrence, in addition to chemotherapy, was one of the better prognostic factors for overall survival [10]. In this case, RT may have played a role. In a non-transplant setting, 64% of adrenal metastases [7] and 53.9% of bone metastases [8] responded. However, at the time of the adrenal metastasis, we did not administer systemic chemotherapy together with RT because systemic chemotherapy was not covered by the national insurance and the response to the first application of RT at the time of the first adrenal metastasis was good. Regarding immunosuppressant, the use of mTORi may prevent HCC recurrence after transplantation [11]. In this case, an mTORi was used to maintain immunosuppression to reduce tacrolimus.
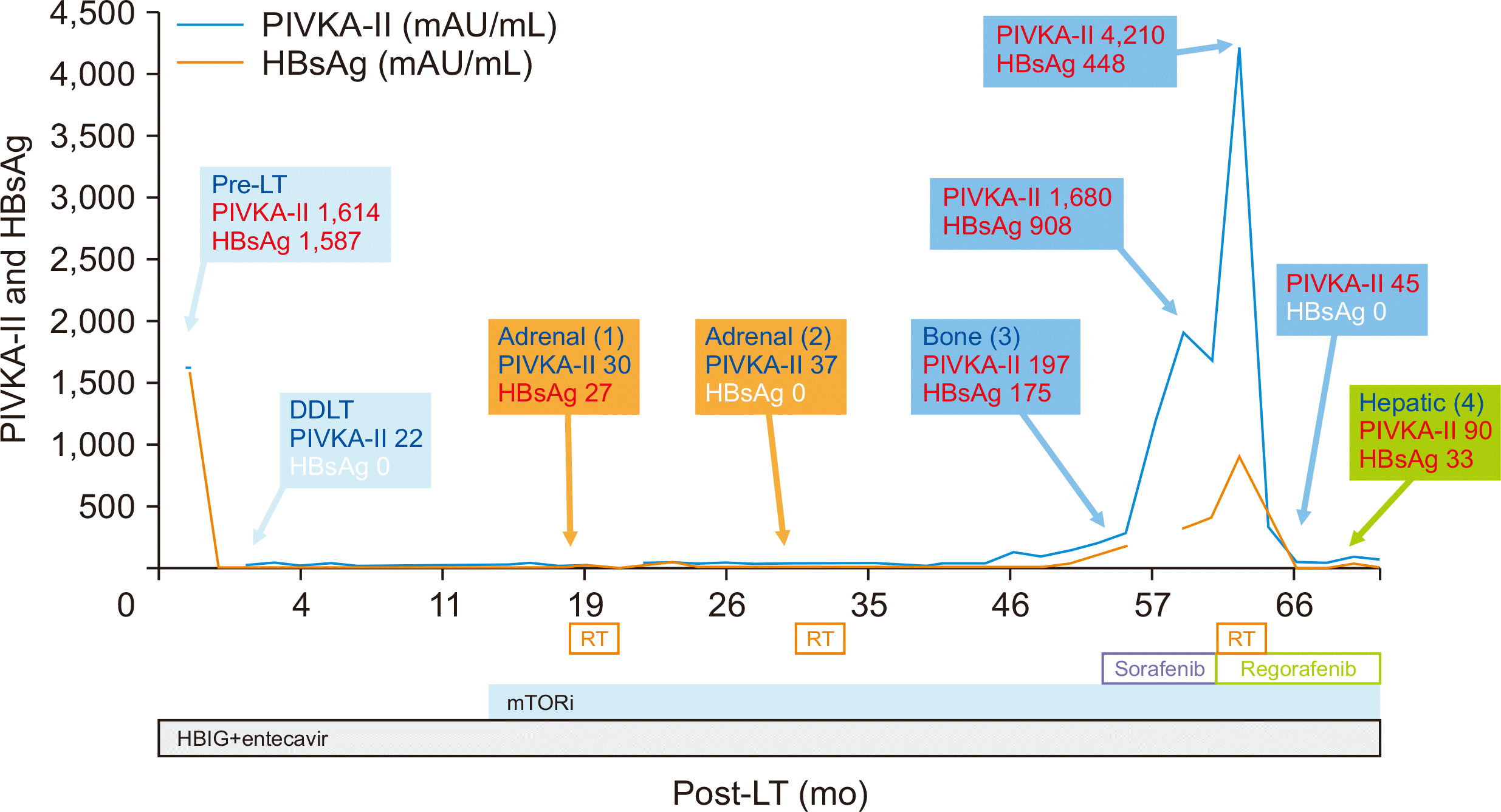
A retrospective study found that 37% of HCC recurrences were accompanied by HB recurrence, and the intended treatment of HCC led to HBsAg clearance in 57% of cases without tumor marker elevation [12]. In this case, HCC and HB recurrences showed similar trends (Fig. 6). Except for the second adrenal metastasis, HCC recurrence was accompanied by HBsAg reappearance. The lesson we learned from this case is that even if tumor markers are normal, recurrence may be suspected if HBsAg becomes positive without graft dysfunction. When HCC was controlled without changes in the HB prophylactic therapy, HB was cleared. This suggests that there is a positive correlation between HB infection and HCC recurrence [13]. Therefore, it can be inferred that HBsAg clearance reflects the HCC treatment response [14].
Before transplantation, the patient was treatment-naive for HBV with a high viral DNA load, and he experienced HCC and HB recurrence after 18 months. The survival periods after transplantation, primary adrenal metastasis, and bone metastasis were 71, 53, and 17 months, respectively. Stable disease status according to the RECIST criteria [6] is defined as more than 9 months with regorafenib combined with RT without graft failure or rejection. Unfortunately, 9 months after combined treatment with regorafenib and RT, graft HCC recurrence was observed.
In conclusion, combination therapy consisting of systemic and locoregional therapies may prolong patient survival, even after HCC recurrence. HB recurrence along with HCC recurrence without graft dysfunction requires no further treatment, but can be used as a surrogate marker for HCC recurrence and treatment responsiveness.
ACKNOWLEDGMENTS
Author Contributions
Conceptualization: JYK, JK, HHC, JL, SYH, SKH, YC, NJY, KWL, KSS. Data curation: JYK, YJK, EKC. Funding acquisition: NJY. Methodology: JYK, NJY, SKH, YC, KWL, KSS. Visualization: all authors. Writing–original draft: JYK, NJY, YJK, EKC. Writing–review & editing: JYK, NJY, JML, SKH, YC, NJY, KWL, KSS. All authors read and approved the final manuscript.
REFERENCES
1. Teegen EM, Mogl MT, Pratschke J, Rayes N. 2018; Adrenal metastasis of hepatocellular carcinoma in patients following liver resection or liver transplantation: experience from a tertiary referral center. Int J Surg Oncol. 2018:4195076. DOI: 10.1155/2018/4195076. PMID: 30151282. PMCID: PMC6087597.
2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. 2022; BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 76:681–93. DOI: 10.1016/j.jhep.2021.11.018. PMID: 34801630. PMCID: PMC8866082.

3. Bhatia R, Ravulapati S, Befeler A, Dombrowski J, Gadani S, Poddar N. 2017; Hepatocellular carcinoma with bone metastases: incidence, prognostic significance, and management-single-center experience. J Gastrointest Cancer. 48:321–5. DOI: 10.1007/s12029-017-9998-6. PMID: 28891006.
4. Chagas AL, Felga GE, Diniz MA, Silva RF, Mattos AA, Silva RC, et al. 2019; Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: clinical profile and prognostic factors of survival. Eur J Gastroenterol Hepatol. 31:1148–56. DOI: 10.1097/MEG.0000000000001448. PMID: 31247632. PMCID: PMC6687037.

5. Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, et al. 2010; Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 16:678–84. DOI: 10.1002/lt.22047. PMID: 20440777.
6. de'Angelis N, Landi F, Carra MC, Azoulay D. 2015; Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 21:11185–98. DOI: 10.3748/wjg.v21.i39.11185. PMID: 26494973. PMCID: PMC4607916.
7. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. 2009; New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–47. DOI: 10.1016/j.ejca.2008.10.026. PMID: 19097774.
8. Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, et al. 2016; Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 263:842–50. DOI: 10.1097/SLA.0000000000001578. PMID: 26779979.
9. Jamtani I, Lee KW, Choi Y, Choi Y, Lee JM, Han ES, et al. 2021; Tailored prediction model of survival after liver transplantation for hepatocellular carcinoma. J Clin Med. 10:2869. DOI: 10.3390/jcm10132869. PMID: 34203396. PMCID: PMC8268829.
10. Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, et al. 2021; Regorafenib efficacy after sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation: a retrospective study. Liver Transpl. 27:1767–78. DOI: 10.1002/lt.26264. PMID: 34388851.
11. Lee KW, Kim SH, Yoon KC, Lee JM, Cho JH, Hong SK, et al. 2020; Sirolimus prolongs survival after living donor liver transplantation for hepatocellular carcinoma beyond milan criteria: a prospective, randomised, open-label, multicentre phase 2 trial. J Clin Med. 9:3264. DOI: 10.3390/jcm9103264. PMID: 33053849. PMCID: PMC7600292.

12. Saab S, Yeganeh M, Nguyen K, Durazo F, Han S, Yersiz H, et al. 2009; Recurrence of hepatocellular carcinoma and hepatitis B reinfection in hepatitis B surface antigen-positive patients after liver transplantation. Liver Transpl. 15:1525–34. DOI: 10.1002/lt.21882. PMID: 19877207.
13. Yi NJ, Suh KS, Cho JY, Kwon CH, Lee KW, Joh JW, et al. 2007; Recurrence of hepatitis B is associated with cumulative corticosteroid dose and chemotherapy against hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 13:451–8. DOI: 10.1002/lt.21043. PMID: 17318862.
14. Yi NJ, Lee KW, Kong SY, Park KU, Lee KB, Hong G, et al. 2013; Outcome of various treatments for posttransplant hepatitis B virus recurrence. World J Surg. 37:812–9. DOI: 10.1007/s00268-013-1914-z. PMID: 23344522.
Fig. 1
Computed tomography of the abdomen revealing hepatocellular carcinoma (approximately 4.7 cm). (A) Atrial phase (arrow), and (B) subtle washout in the delayed phase (arrow).
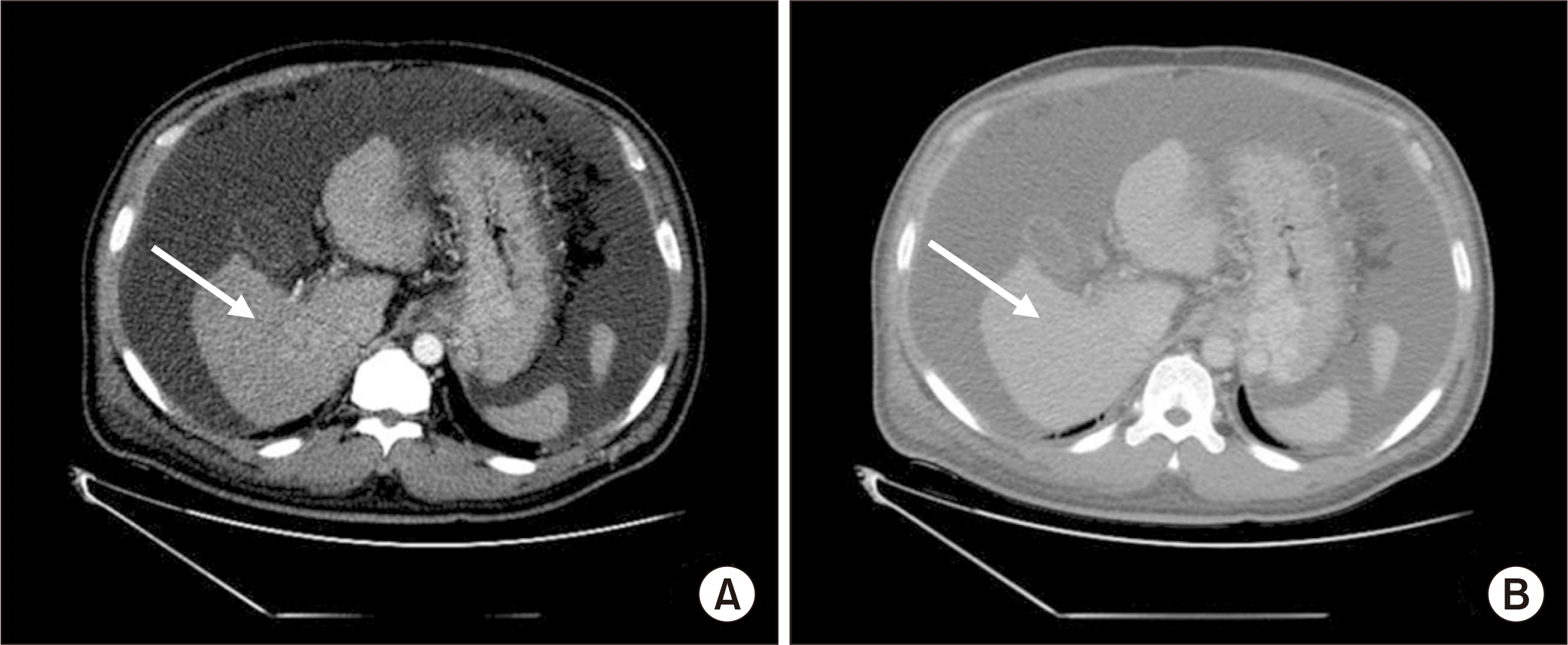
Fig. 2
Time flow of treatment after liver transplantation and hepatocellular carcinoma (HCC) and hepatitis B virus (HBV) recurrence. DDLT, deceased donor liver transplantation; ES w II/m II, Edmonson-Steiner nuclear grade worst II/major II; PIVKA-II, protein induced by vitamin K absence or antagonist-II; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; RT, radiotherapy; IS, immunosuppressant; mTORi, mammalian target of rapamycin inhibitor; HBIG, HB immunoglobulin. a)Regorafenib 160 mg/day to 120 mg/day to 80 mg/day; b)Lipiodol 2 mL, adriamycin 10 mg.
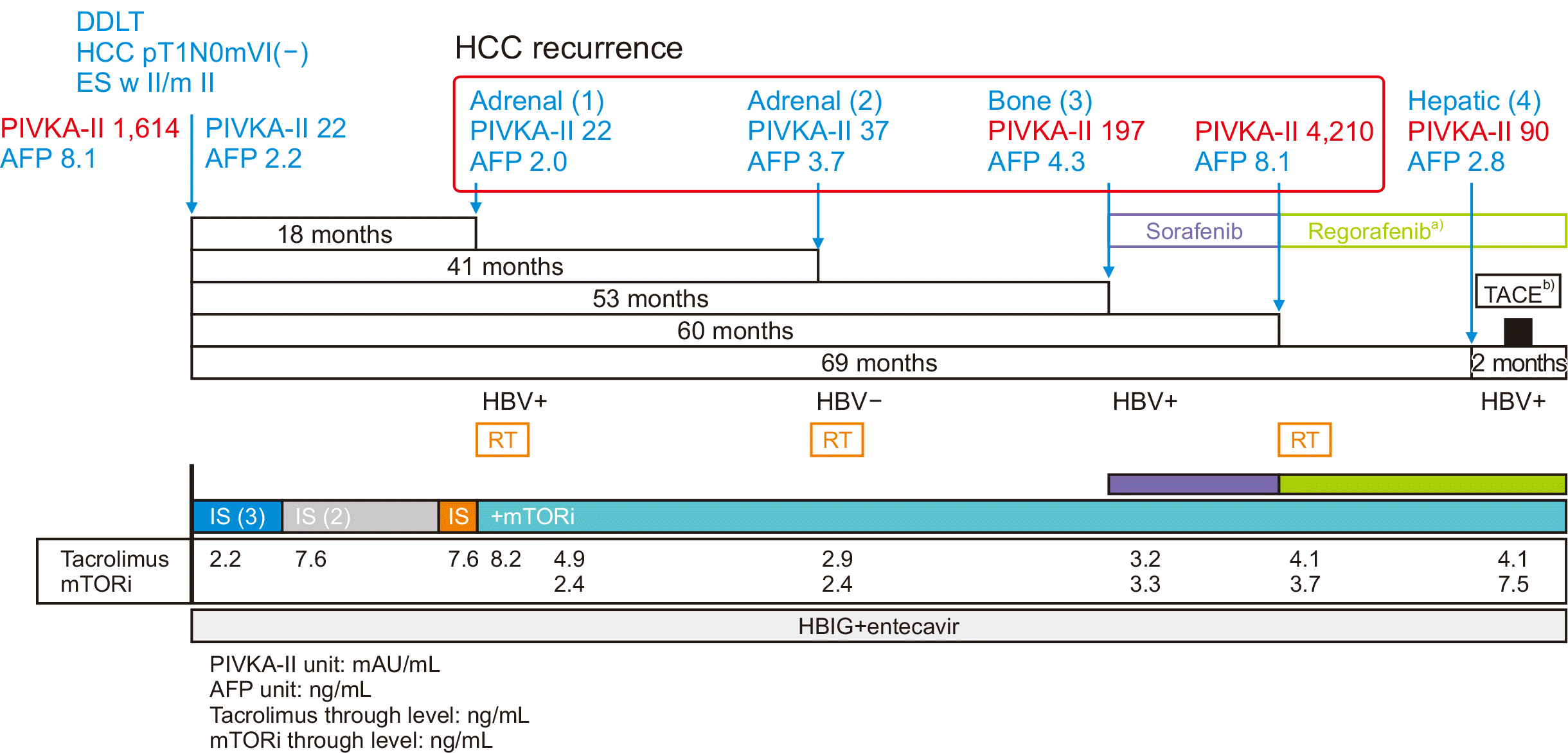
Fig. 3
Hepatocellular carcinoma (HCC) recurrence in the right adrenal gland 18 months after transplantation. (A) Computed tomography (CT) scan of the abdomen revealing right adrenal metastasis of HCC (arrow). (B) Mild uptake in the right adrenal gland on positron emission tomography CT scans (arrow). CT scans with (C) arterial phase and (D) portal phase revealed recurrence in the right adrenal gland (arrow), 12 months after the first treatment for metastasis and 41 months after transplantation.
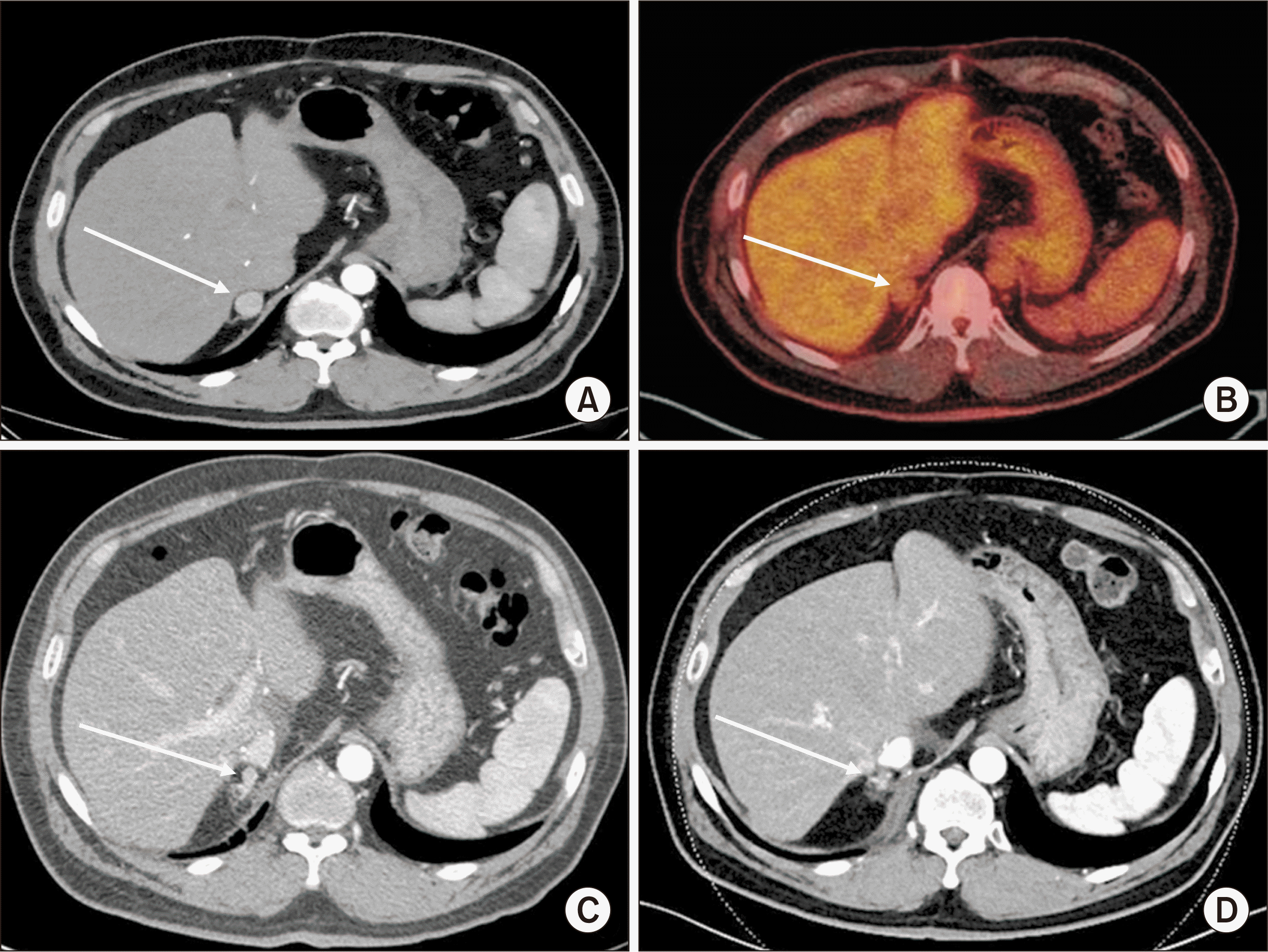
Fig. 4
Bone metastasis at the fourth lumbar pedicle 17 months later after the second recurrence (A) computed tomography (CT) revealed bone metastasis at the fourth lumbar pedicle (arrow). (B) On magnetic resonance imaging (MRI), bone metastasis at the lumbar pedicle progressed on sorafenib treatment (arrow). (C) On CT, adrenal lesions were stable 14 months after bone recurrence (arrow). (D) On MRI, the lumbar lesion was stable for 14 months after bone recurrence (arrow).
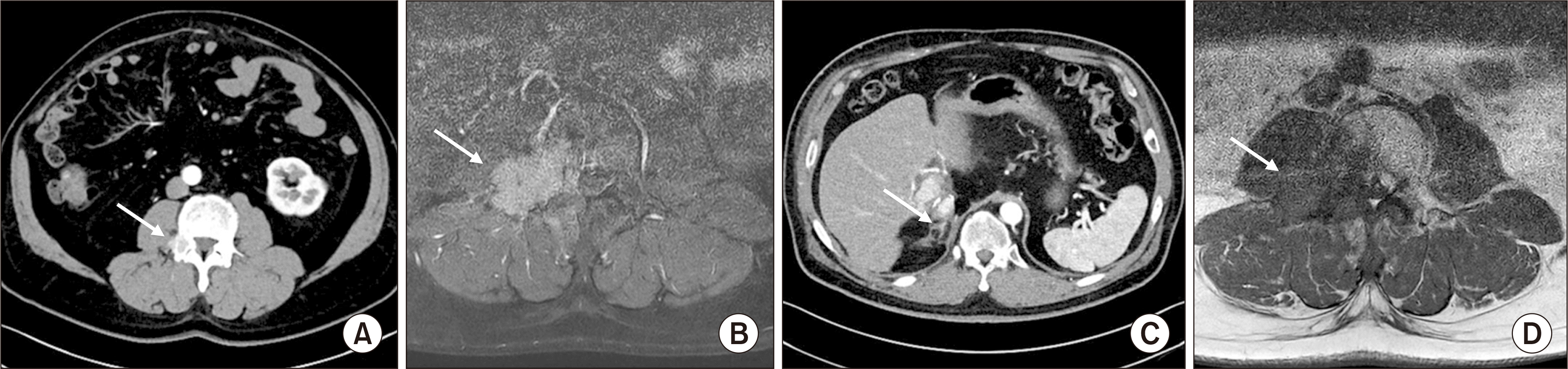
Fig. 5
Hepatocellular carcinoma metastasis in the liver 17 months after the third recurrence. Computed tomography revealed hepatic recurrence (A) at S7 (segment 7; arrow) and (B) at S3 (arrow). Magnetic resonance imaging scans revealed hepatic recurrence (C) at S7 (arrow) and (D) at S7 (arrow), (E) two lesions at S4 and S3 (arrows), and (F) one more at S3 (arrow).
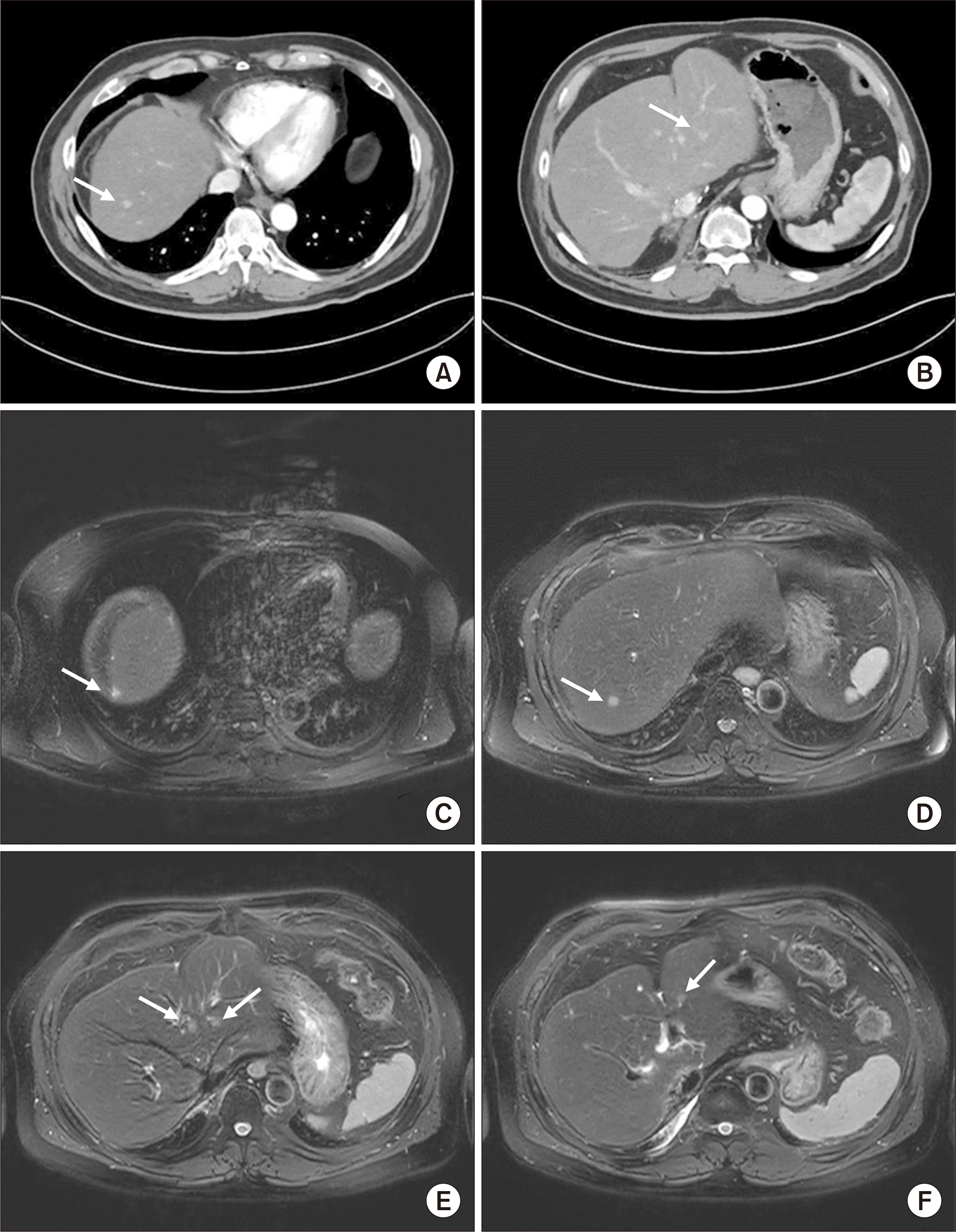
Fig. 6
Hepatocellular carcinoma (HCC) recurrence and treatment, protein induced by vitamin K absence or antagonist-II (PIVKA-II) levels and hepatitis B surface antigen (HBsAg) titers. HCC and hepatitis B recurrences showed similar trends. LT, liver transplantation; DDLT, deceased donor LT; RT, radiotherapy; mTORi, mammalian target of rapamycin inhibitor; HBIG, HB immunoglobulin.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download