Abstract
Objective
The sleep quality is worse in rheumatoid arthritis (RA) patients than in healthy controls and it is more difficult to achieve a satisfactory quality of life after treatment with age. Our aim is to assess the quality of life and sleep in elderly onset RA patients and to analyze the effect of disease-modifying agents on sleep and quality of life.
Methods
Thirty-four older patients with RA patients and 30 healthy controls are included in the study. Sleep quality was evaluated with the Pittsburg sleep quality index and quality of life with Short Form-36. Parametric/non-parametric tests and Spearman/Pearson correlation analysis were applied for the data according to the distribution.
Results
While the rate of poor sleep quality before treatment was 67.6%, the rate was 26.5% after treatment. There was a statistically significant difference before and after treatment in terms of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, and scores for sleep disturbance. The mean steroid dose and Disease Activity Score-28 were higher in patients with poor sleep quality than in patients with good sleep quality. Patients with poor sleep quality had lower mean physical function, pain, general health, social function, emotional role difficulties, and energy/vitality values than patients with good sleep quality.
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune, and multisystemic rheumatic disease that affects joints, many organs, and systems. Its prevalence increases with aging and the disease is seen in 2% of the older patients [1-3]. Late-onset RA is defined as RA that occurs in individuals over the age of 60~65 years [4]. Clinically, while early-onset RA has an insidious onset, late-onset RA appears more acute. Large joints such as the shoulder are affected in late-onset RA compared to early-onset RA [5]. In terms of clinical findings, comorbid diseases are more common in late-onset RA compared to early-onset RA [6,7]. RA has significantly worse consequences on physical functioning [8]. The disease significantly affects social relations, family life, and psychological state [8]. Patients may have difficulty in fulfilling their daily duties, may have to change occupations, or may retire early. Changes in self-perception associated with painful stimuli, reduced functional ability, labor, and social disability can also cause emotional and mental disorders.
Changes occur in biochemical parameters, pharmacokinetics/pharmacodynamics of drugs, functions of immune system cells, and increases in comorbid diseases along with aging. During aging, changes are observed in physical, biological, and social roles in people. RA in older people has a lower physical function, social functionality, and emotionality than adults [9]. Both psychological and sleep problems affect the quality of life in patients with RA. Sleep is important for preserving and renewing the energy that leads to the rejuvenation of the body and mind, and for strengthening the memory [10]. Approximately 70% of patients with RA have daytime sleepiness, difficulty falling asleep, and maintaining sleep [11]. It is thought that changes in pro-inflammatory cytokines and melatonin levels contribute to sleep disorders in RA [12].
High disease activity affects the physical and mental quality of life [13]. It has been reported that sleep quality is worse in RA patients than healthy controls and it is more difficult to achieve a satisfactory quality of life after treatment with aging [14]. Data on sleep and quality of life in older patients with RA are scarce. Our aim in this study is to evaluate sleep and quality of life in older patients with RA and to investigate the effects of disease-modifying agents on sleep and quality of life.
The study included 34 RA patients diagnosed older than 65 years and 30 healthy controls. Demographic data such as age, sex, marital status, education and employment status, smoking, lung involvenment, rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP) were recorded. Sleep quality was assessed with the Pittsburgh Sleep Quality Index and quality of life with the Short Form-36 (SF-36). There are seven components in the Pittsburgh Sleep Quality Index, each scored from 0 (no difficulty) to 3 (severe difficulty). Higher scores indicate worse sleep quality. A total Pittsburgh Sleep Quality Index score >5 was considered as poor sleep quality [15]. In SF-36, each domain’s scores range from 0 to 100, with a higher score describing a more positive state of health. Examination, laboratory values, sleep, and quality of life of the patients were evaluated before and 6 months after the diagnosis and treatment of RA patients. We excluded patients with connective tissue disease other than RA, those with a history of infection and malignancy, and patients under 65 years of age. The study protocol was approved by the Faculty of Medicine Ethics Committee of Aydin Adnan Menderes University and designed consistent with the Declaration of Helsinki (approval number: E.43227).
Data were evaluated using SPSS 21.0 (IBM Co., Armonk, NY, USA). Descriptive statistics were given as mean±standard deviation, median (25~75p), frequency (n), and percentage (%). Kolmogorov–Smirnov test, Mann–Whitney U test, Wilcoxon signed-rank test, independent sample t-test, and paired-samples t-test were used for statistical analyses. A chi-squared test was used to compare qualitative data. Spearman and Pearson correlation analysis was used to determine whether there was a linear relationship between parameters. If the correlation coefficient (r) is <0.2, it is very weak, between 0.2~0.4 weak, between 0.4~0.6 moderate, between 0.6~0.8 high, and >0.8 was considered as very high correlation. Regression analysis was applied to evaluate the relationship between two or more quantitative variables. The data were calculated with a 95% confidence interval, and a p-value <0.05 was considered statistically significant.
The mean age of the patients with a diagnosis of RA included in the study was 72.8±6.7 years, 23 of them were female and 11 of them were male. Smoking was present in 14.7% of the patients. In the control group, the mean age was 70.5±4.4 years, 9 of which were male and 21 were female. All RA patients were newly diagnosed and the duration of treatment was the same for each patient. There was no difference between RA and control groups in terms of age, gender, smoking/alcohol use, marital status, employment status, and educational status. The median total Pittsburgh Sleep Quality Index 15 in older RA patients before treatment. Total Pittsburgh Sleep Quality Index scores, physical and mental component scores were lower between the RA and control groups (p<0.001).
Sleep quality was good in 32.4% of older patients with RA before treatment. In terms of sleep quality, there was no statistically significant difference between good and poor sleep quality groups in terms of age, sex, smoking-alcohol use, marital status, employment status, education status, organ involvement, RF, anti-CCP. There were no patients using nonsteroidal anti-inflammatory drugs. Of the patients with good sleep quality in the 6th month, 76% used methotrexate, 24% leflunomide, and 60% hydroxychloroquine. There was no statistically significant difference between the two groups in terms of the use of disease-modifying antirheumatic drugs (DMARDs). Conventional synthetic DMARDs were used safely in the older patients with RA and no side effects have been observed. The mean steroid dose and Disease Activity Score-28 (DAS-28) were higher in patients with poor sleep quality than in patients with good sleep quality. The mean DAS-28 was 4.8±0.5 in patients with good sleep quality and 5.4±0.9 in patients with poor sleep quality. It was 3.3±1.4 and 4.1±1.3 after DMARD treatment, respectively. There were no significant differences in DAS-28 between sleep groups. The mean DAS-28 was 5.2 before the treatment, and it reached 3.5 after treatment (p<0.001). There were significant differences in steroid dose between groups before treatment (13.8±10.3 vs. 20.8±10.7 mg/day, p=0.008). Although the mean steroid dose was higher in patients with poor sleep quality, no significant differences were found after treatment (6.8±5.5 vs. 8.4±5.0 mg/day, p>0.05) (Table 1).
The rate of patients with poor sleep quality after treatment was found to be 26.5%. There was a statistically significant difference before and after treatment in terms of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, and sleep disorder scores (p<0.01) (Figure 1). The physical function, physical role difficulty, pain, general health and social function, emotional role difficulty, mental health, and energy/vitality scores, which are physical components evaluated with SF-36 increased after treatment in RA patients. There was a statistically significant difference between before and after treatment in terms of physical function, physical role difficulty, pain, emotional role difficulty, and energy/vitality (p<0.05) (Figure 2).
A negative correlation was found between Pittsburgh Sleep Quality Index and quality of life scores before and after treatment. There was a statistically significant correlation between physical role difficulty and Pittsburgh Sleep Quality Index components such as sleep duration, sleep efficiency, sleep disturbance, and the total score. Also, a negative correlation was found between DAS-28 and physical function, physical role difficulty, pain, general health, social function, emotional role difficulty, mental health, energy/vitality before and after treatment. In addition, statistically significant correlations were found between physical function and sleep efficiency, physical role difficulty and sleep efficiency, emotional role difficulty and subjective sleep quality, energy/vitality and sleep disturbance after treatment (Table 2).
Sleep problems are generally associated with physiological and psychosocial problems in RA. The patients mainly have pain-related sleep problems. RA patients with insomnia problems have more problems in their daily lives and general health areas. Sleep problems gradually decrease the quality of life in these patients. In addition, psychological problems such as not being able to fall asleep, continuous interruption of night sleep, daytime sleepiness, anxiety, and depression affect the quality of life in these patients [16]. Subjective sleep, one of the components of the Pittsburgh Sleep Quality Index, shows the sleep quality that varies from person to person. Apart from RA, there are also sleep problems in various rheumatic diseases. High disease activity of ankylosing spondylitis is associated with poor sleep quality. A positive correlation was reported between the ankylosing spondylitis disease activity indices Bath Ankylosing Spondylitis Disease Activity Index, Ankylosing Spondylitis Disease Activity Score with ESR, Ankylosing Spondylitis Disease Activity Score with CRP and sleep quality [17]. Pittsburgh Sleep Quality Index was developed in 1989 by Buysse et al. [15] and Turkish validity and reliability study was performed by Ağargün et al. [18]. A total score >5 was considered as poor sleep quality [15,18]. The cutoff value may be different depending on the language. According to the reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index, the sensitivity and specificity to distinguish poor and good sleepers were 0.943 and 0.844 using the best cutoff point of 8.5 [19]. Also, The Korean version of the Pittsburgh Sleep Quality Index was used to evaluate the relationship between disease activity, depression, and quality of life for Behçet’s disease in the Korean population [20]. Sleep latency shows the time to fall asleep, sleep efficiency shows sleep quality, and daytime functionality shows how much trouble sleep causes in performing normal activities during the day [21]. A significant difference was found between the patient with RA and control groups in terms of subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, and daytime dysfunction components [22]. In another study conducted with RA patients, sleep latency, habitual sleep efficiency, sleep disturbance, and global Pittsburgh Sleep Quality Index scores were found to be statistically significantly higher in RA patients compared to the control group [23].
Sleep quality is affected by demographic characteristics, daily activities, smoking/alcohol use, environmental factors, and DMARDs. In a study investigating the effect of infliximab on sleep quality, improvement in sleep disorders was reported after infliximab infusion [24]. This was thought to be related to a decrease in tumor necrosis factor (TNF)-alpha levels in the blood rather than a decrease in joint pain. In another study, a decrease in disease activity and an increase in sleep efficiency were found with TNF-alpha blocking agents [25]. Contrary to these, there are also studies showing that RA treatment does not affect the Pittsburgh Sleep Quality Index [25,26]. In our study, the rate in the older patients group with good sleep quality was 32.4% before treatment and a Pittsburgh Sleep Quality Index score of >5, that is, poor sleep quality was 67.6%. After the treatment, the rate of patients with poor sleep quality decreased to 26.5%. In addition, a statistically significant difference was found before and after treatment in the scores of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, and sleep disorder. And, in the follow-up of our older patients, no side effects associated were observed with the use of DMARDs. It has been reported that sleep disturbance and mood are associated with disease activity in RA patients [21]. And, a positive correlation was found between DAS-28 and Pittsburgh sleep quality scale scores in RA patients [23]. Also, in another study, a positive correlation was found between DAS-28 and Pittsburgh sleep quality scale score, while a negative correlation was found between the Epworth sleep scale score and DAS-28 [27]. In our study, the DAS-28 was higher in patients with poor sleep quality compared to good sleep quality both before and after treatment. There was a positive correlation between DAS-28 and Pittsburgh Sleep Quality Index both before and after treatment.
RA has significantly worse consequences on physical functioning. The disease significantly affects social relations, family life, and psychological state. Patients may have difficulty in fulfilling their daily duties, may have to change occupations, or may retire early. Painful stimuli decreased functional ability, changes in self-perception associated with labor and social inadequacy may also cause emotional and mental disorders [9]. In a study comparing the quality of life of fibromyalgia, RA, AS and healthy volunteers, the mean physical and mental component scores in all three groups were found to be lower than those of healthy volunteers [28]. The group with the lowest physical component was found to be RA, and the group with the lowest mental component was fibromyalgia. In the study, it was concluded that the disease affects the quality of life negatively in all 3 patient groups [28]. In a study evaluating sleep problems in fibromyalgia and RA, more depression, pain, and insomnia-related symptoms were reported in fibromyalgia patients than in RA patients [29]. The limitation of the study is that we could not evaluate fibromyalgia and its relationship with sleep disturbance and quality of life in our patients. In another study, all components of the SF-36 index except the physical role score were found to be lower than those of osteoarthritis [30]. In a study evaluating the quality of life with SF-36 in females aged 15~49 years, it was reported that physical role difficulties, pain, general health, vitality, emotional role difficulties, and mental health components were lower, while physical function and social functions were higher [31]. In our study, there was a negative correlation between age and quality of life components evaluated with SF-36. So, as the age increased, the scores of the quality of life components and the quality of life decreased. However, there was a significant increase in quality of life scores with treatment.
As a result, both sleep and quality of life are worse in older patients with RA before and after treatment compared to the healthy control group. In addition, aging and high disease activity are correlated with poor sleep and quality of life in RA patients. After the treatment, improvement in sleep and quality of life was observed in the patients. Again, conventional synthetic DMARDs are used safely in the older patients with RA and no side effects have been observed. Older patients with RA should be regularly evaluated in terms of sleep and quality of life, and appropriate treatment should be provided.
REFERENCES
1. Sparks JA. 2019; Rheumatoid arthritis. Ann Intern Med. 170:ITC1–16. DOI: 10.7326/AITC201901010. PMID: 30596879.

2. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. 2018; Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6:15. DOI: 10.1038/s41413-018-0016-9. PMID: 29736302. PMCID: PMC5920070.

3. Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, et al. 2018; Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. 27:501–7. DOI: 10.1159/000493390. PMID: 30173215. PMCID: PMC6422329.
4. Kobak S, Bes C. 2018; An autumn tale: geriatric rheumatoid arthritis. Ther Adv Musculoskelet Dis. 10:3–11. DOI: 10.1177/1759720X17740075. PMID: 29290762. PMCID: PMC5724645.
5. Tan TC, Gao X, Thong BY, Leong KP, Lian TY, Law WG, et al. TTSH Rheumatoid Arthritis Study Group. 2017; Comparison of elderly- and young-onset rheumatoid arthritis in an Asian cohort. Int J Rheum Dis. 20:737–45. DOI: 10.1111/1756-185X.12861. PMID: 27135312.
6. Zhang JF, Ye XL, Duan M, Zhou XL, Yao ZZ, Zhao JX. 2020; [Clinical characteristics of elderly and younger onset rheumatoid arthritis]. Zhonghua Yi Xue Za Zhi. 100:3788–92. Chinese.
7. Kłodziński Ł, Wisłowska M. 2018; Comorbidities in rheumatic arthritis. Reumatologia. 56:228–33. DOI: 10.5114/reum.2018.77974. PMID: 30237627. PMCID: PMC6142024.
8. Martinec R, Pinjatela R, Balen D. 2019; Quality of life in patients with rheumatoid arthritis - a preliminary study. Acta Clin Croat. 58:157–66. DOI: 10.20471/acc.2019.58.01.20. PMID: 31363338. PMCID: PMC6629210.
9. Roma I, Almeida ML, Mansano NS, Viani GA, Assis MR, Barbosa PMK. 2014; [Quality of life in adults and elderly patients with rheumatoid arthritis]. Rev Bras Reumatol. 54:279–86. Portuguese. DOI: 10.1016/j.rbre.2014.03.025.

10. Worley SL. 2018; The extraordinary importance of sleep: the detrimental effects of inadequate sleep on health and public safety drive an explosion of sleep research. P T. 43:758–63.
11. Grabovac I, Haider S, Berner C, Lamprecht T, Fenzl KH, Erlacher L, et al. 2018; Sleep quality in patients with rheumatoid arthritis and associations with pain, disability, disease duration, and activity. J Clin Med. 7:336. DOI: 10.3390/jcm7100336. PMID: 30304765. PMCID: PMC6210607.
12. MacDonald IJ, Huang CC, Liu SC, Tang CH. 2020; Reconsidering the role of melatonin in rheumatoid arthritis. Int J Mol Sci. 21:2877. DOI: 10.3390/ijms21082877. PMID: 32326031. PMCID: PMC7215432.
13. Mangoni AA, Jackson SH. 2004; Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 57:6–14. DOI: 10.1046/j.1365-2125.2003.02007.x. PMID: 14678335. PMCID: PMC1884408.
14. Son CN, Choi G, Lee SY, Lee JM, Lee TH, Jeong HJ, et al. 2015; Sleep quality in rheumatoid arthritis, and its association with disease activity in a Korean population. Korean J Intern Med. 30:384–90. DOI: 10.3904/kjim.2015.30.3.384. PMID: 25995669. PMCID: PMC4438293.
15. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. 1989; The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28:193–213. DOI: 10.1016/0165-1781(89)90047-4. PMID: 2748771.
16. Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. 2014; The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 44:123–30. DOI: 10.1016/j.semarthrit.2014.05.001. PMID: 24973898.
17. Song BW, Jeong HJ, Kim BY, Cho YW, Son CN, Kim SS, et al. 2021; Bath ankylosing spondylitis disease activity index is associated with the quality of sleep in ankylosing spondylitis patients. J Rheum Dis. 28:143–9. DOI: 10.4078/jrd.2021.28.3.143.
18. Ağargün MY, Kara H, Anlar Ö. 1996; [The validity and reliability of the Pittsburgh Sleep Quality Index]. Turk Psikiyatri Derg. 7:107–15. Turkish.
19. Sohn SI, Kim DH, Lee MY, Cho YW. 2012; The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 16:803–12. DOI: 10.1007/s11325-011-0579-9. PMID: 21901299.
20. Lee J, Kim SS, Jeong HJ, Son CN, Kim JM, Cho YW, et al. 2017; Association of sleep quality in Behcet disease with disease activity, depression, and quality of life in Korean population. Korean J Intern Med. 32:352–9. DOI: 10.3904/kjim.2016.367. PMID: 28192886. PMCID: PMC5339476.
21. Faulkner S, Sidey-Gibbons C. 2019; Use of the Pittsburgh Sleep Quality Index in people with schizophrenia spectrum disorders: a mixed methods study. Front Psychiatry. 10:284. DOI: 10.3389/fpsyt.2019.00284. PMID: 31143131. PMCID: PMC6520598.
22. Goes ACJ, Reis LAB, Silva MBG, Kahlow BS, Skare TL. 2017; Rheumatoid arthritis and sleep quality. Rev Bras Reumatol Engl Ed. 57:294–8. DOI: 10.1016/j.rbr.2016.06.002. PMID: 28743355.
23. Guo G, Fu T, Yin R, Zhang L, Zhang Q, Xia Y, et al. 2016; Sleep quality in Chinese patients with rheumatoid arthritis: contributing factors and effects on health-related quality of life. Health Qual Life Outcomes. 14:151. DOI: 10.1186/s12955-016-0550-3. PMID: 27852301. PMCID: PMC5111274.
24. Zamarrón C, Maceiras F, Mera A, Gómez-Reino JJ. 2004; Effect of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis. 63:88–90. DOI: 10.1136/ard.2003.007831. PMID: 14672898. PMCID: PMC1754718.
25. Taylor-Gjevre RM, Gjevre JA, Nair BV, Skomro RP, Lim HJ. 2011; Improved sleep efficiency after anti-tumor necrosis factor α therapy in rheumatoid arthritis patients. Ther Adv Musculoskelet Dis. 3:227–33. DOI: 10.1177/1759720X11416862. PMID: 22870481. PMCID: PMC3383532.
26. Sariyildiz MA, Batmaz I, Bozkurt M, Bez Y, Cetincakmak MG, Yazmalar L, et al. 2014; Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res. 6:44–52. DOI: 10.4021/jocmr1648w. PMID: 24400031. PMCID: PMC3881989.
27. Westhovens R, Van der Elst K, Matthys A, Tran M, Gilloteau I. 2014; Sleep problems in patients with rheumatoid arthritis. J Rheumatol. 41:31–40. DOI: 10.3899/jrheum.130430. PMID: 24293569.
28. Walker EA, Keegan D, Gardner G, Sullivan M, Katon WJ, Bernstein D. 1997; Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: I. Psychiatric diagnoses and functional disability. Psychosom Med. 59:565–71. DOI: 10.1097/00006842-199711000-00002. PMID: 9407573.
29. Belt NK, Kronholm E, Kauppi MJ. 2009; Sleep problems in fibromyalgia and rheumatoid arthritis compared with the general population. Clin Exp Rheumatol. 27:35–41.
30. Doğan A, Ceceli E, Okumuş M, Gökkaya NKO, Kutsal YG, Borman P, et al. 2011; [Identifying the characteristics of geriatric patients who referred to outpatient clinics of physical medicine and rehabilitation: a multicenter descriptive study]. Turk J Phys Med Rehabil. 57:143–9. Turkish.
31. Bulut İ, Deveci SE. 2017; [The life quality of women aged 15-49 living in Elazig city centre and the factors affecting it]. Firat Üniv Sağlık Bilim Tıp Derg. 31:61–9. Turkish.
Fig. 1
Distribution of scores according to Pittsburgh Sleep Quality Index of patients with rheumatoid arthritis before and after treatment. Wilcoxon signed-rank test; *p<0.01.
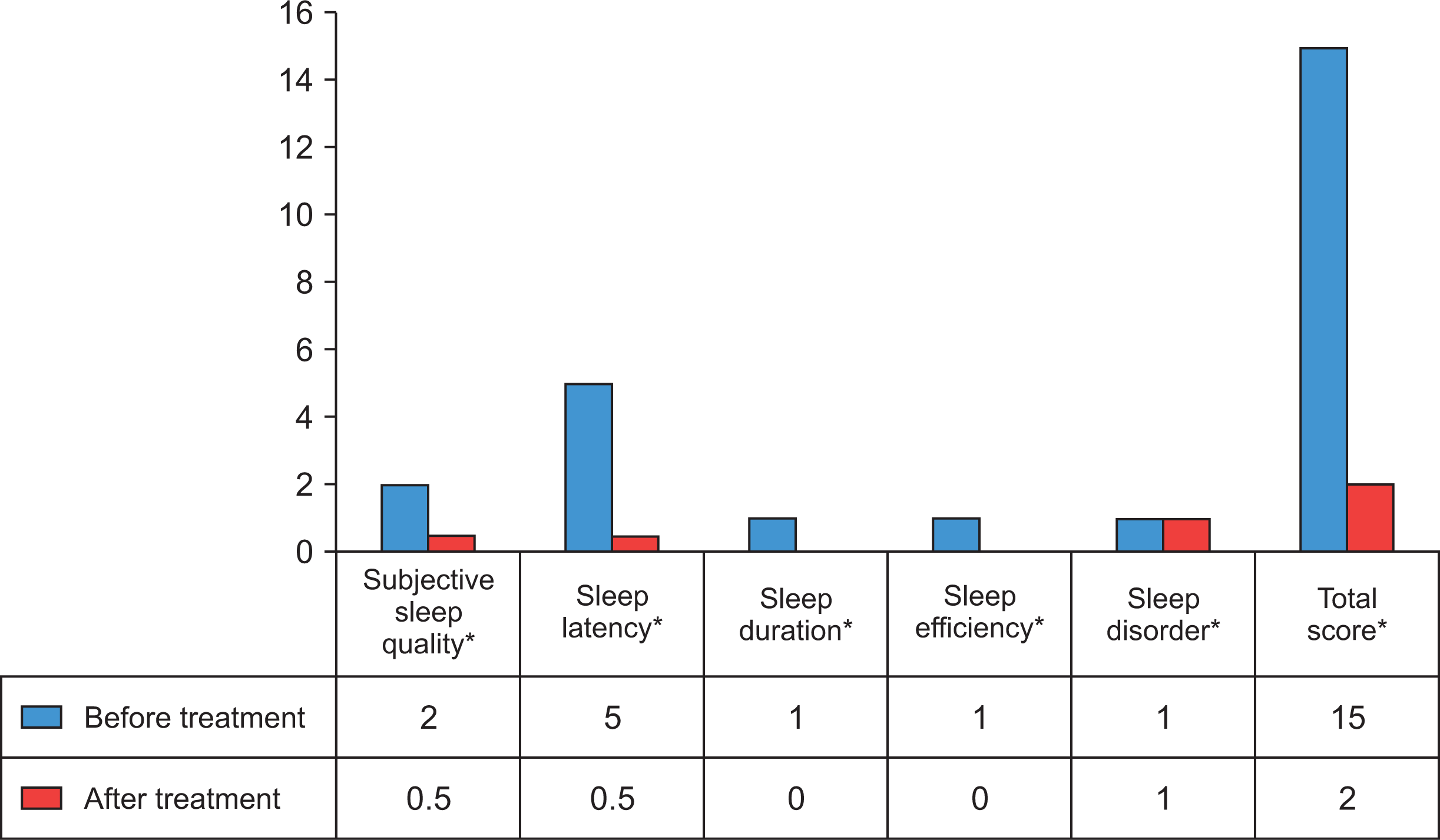
Fig. 2
Distribution of scores according to quality of life evaluated with SF-36 (Short Form-36) in patients with rheumatoid arthritis before and after treatment. Paired-samples t-test; *p<0.05.

Table 1
Demographic and laboratory characteristics of rheumatoid arthritis patients with good and poor sleep quality before treatment
Table 2
The correlation between the quality of life index and Pittsburgh Sleep Quality Index components in patients with rheumatoid arthritis on the 6th month of treatment




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download