3. Dehlin M, Jacobsson L, Roddy E. 2020; Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 16:380–90. DOI:
10.1038/s41584-020-0441-1. PMID:
32541923.
4. Choi HK, McCormick N, Yokose C. 2022; Excess comorbidities in gout: the causal paradigm and pleiotropic approaches to care. Nat Rev Rheumatol. 18:97–111. DOI:
10.1038/s41584-021-00725-9. PMID:
34921301.

5. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. 2008; Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 28:437–43. DOI:
10.1592/phco.28.4.437. PMID:
18363527. PMCID:
PMC2737273.
6. Zhu Y, Pandya BJ, Choi HK. 2011; Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 63:3136–41. DOI:
10.1002/art.30520. PMID:
21800283.
7. Otaki Y, Konta T, Ichikawa K, Fujimoto S, Iseki K, Moriyama T, et al. 2021; Possible burden of hyperuricaemia on mortality in a community-based population: a large-scale cohort study. Sci Rep. 11:8999. DOI:
10.1038/s41598-021-88631-8. PMID:
33903733. PMCID:
PMC8076257.
8. Koto R, Nakajima A, Horiuchi H, Yamanaka H. 2021; Real-world treatment of gout and asymptomatic hyperuricemia: a cross-sectional study of Japanese health insurance claims data. Mod Rheumatol. 31:261–9. DOI:
10.1080/14397595.2020.1784556. PMID:
32552370.
9. Chuang SY, Lee SC, Hsieh YT, Pan WH. 2011; Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993-1996 to 2005-2008. Asia Pac J Clin Nutr. 20:301–8.
10. Ryu S, Chang Y, Zhang Y, Kim SG, Cho J, Son HJ, et al. 2012; A cohort study of hyperuricemia in middle-aged South Korean men. Am J Epidemiol. 175:133–43. DOI:
10.1093/aje/kwr291. PMID:
22156041.
11. Kim EH, Jeon K, Park KW, Kim HJ, Ahn JK, Jeon CH, et al. 2004; The prevalence of gout among hyperuricemic population. J Korean Rheum Assoc. 11:7–13.
12. Hong SJ, Kim YS, Kim HS. 2012; Prevalence and clinical features of hyperuricemia in Gwangju and Jeonnam territories. J Rheum Dis. 19:138–46. DOI:
10.4078/jrd.2012.19.3.138.
13. Kim Y, Kang J, Kim GT. 2018; Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol. 37:2529–38. DOI:
10.1007/s10067-018-4130-2. PMID:
29790110.
14. Koo BS, Jeong HJ, Son CN, Kim SH, Kim HJ, Kim GH, et al. 2021; Distribution of serum uric acid levels and prevalence of hyper- and hypouricemia in a Korean general population of 172,970. Korean J Intern Med. 36(Suppl 1):S264–72. DOI:
10.3904/kjim.2020.116. PMID:
33227843. PMCID:
PMC8009145.
15. Rai SK, Aviña-Zubieta JA, McCormick N, De Vera MA, Shojania K, Sayre EC, et al. 2017; The rising prevalence and incidence of gout in British Columbia, Canada: population-based trends from 2000 to 2012. Semin Arthritis Rheum. 46:451–6. DOI:
10.1016/j.semarthrit.2016.08.006. PMID:
28040245. PMCID:
PMC5315679.
16. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. 2019; Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 71:991–9. DOI:
10.1002/art.40807. PMID:
30618180. PMCID:
PMC6536335.
17. Anagnostopoulos I, Zinzaras E, Alexiou I, Papathanasiou AA, Davas E, Koutroumpas A, et al. 2010; The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord. 11:98. DOI:
10.1186/1471-2474-11-98. PMID:
20504294. PMCID:
PMC2890601.
18. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. 2015; Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 74:661–7. DOI:
10.1136/annrheumdis-2013-204463. PMID:
24431399. PMCID:
PMC4392307.
19. Zobbe K, Prieto-Alhambra D, Cordtz R, Højgaard P, Hindrup JS, Kristensen LE, et al. 2019; Secular trends in the incidence and prevalence of gout in Denmark from 1995 to 2015: a nationwide register-based study. Rheumatology (Oxford). 58:836–9. DOI:
10.1093/rheumatology/key390. PMID:
30590724.
20. Klemp P, Stansfield SA, Castle B, Robertson MC. 1997; Gout is on the increase in New Zealand. Ann Rheum Dis. 56:22–6. DOI:
10.1136/ard.56.1.22. PMID:
9059136. PMCID:
PMC1752259.
21. Tu FY, Lin GT, Lee SS, Tung YC, Tu HP, Chiang HC. 2015; Prevalence of gout with comorbidity aggregations in southern Taiwan. Joint Bone Spine. 82:45–51. DOI:
10.1016/j.jbspin.2014.07.002. PMID:
25238950.
22. Huang J, Ma ZF, Zhang Y, Wan Z, Li Y, Zhou H, et al. 2020; Geographical distribution of hyperuricemia in mainland China: a comprehensive systematic review and meta-analysis. Glob Health Res Policy. 5:52. DOI:
10.1186/s41256-020-00178-9. PMID:
33292806. PMCID:
PMC7708223.
23. Kuo CF, Grainge MJ, Zhang W, Doherty M. 2015; Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 11:649–62. DOI:
10.1038/nrrheum.2015.91. PMID:
26150127.
24. Hakoda M, Kasagi F. 2019; Increasing trend of asymptomatic hyperuricemia under treatment with urate-lowering drugs in Japan. Mod Rheumatol. 29:880–4. DOI:
10.1080/14397595.2018.1519149. PMID:
30175646.
25. Lee CH, Sung NY. 2011; The prevalence and features of Korean gout patients using the National Health Insurance Corporation database. J Rheum Dis. 18:94–100. DOI:
10.4078/jrd.2011.18.2.94.
26. Kim JW, Kwak SG, Lee H, Kim SK, Choe JY, Park SH. 2017; Prevalence and incidence of gout in Korea: data from the national health claims database 2007-2015. Rheumatol Int. 37:1499–506. DOI:
10.1007/s00296-017-3768-4. PMID:
28676911.
27. Park JS, Kang M, Song JS, Lim HS, Lee CH. 2020; Trends of gout prevalence in South Korea based on medical utilization: a National Health Insurance Service database (2002~2015). J Rheum Dis. 27:174–81. DOI:
10.4078/jrd.2020.27.3.174.
28. Choi HK, Ford ES, Li C, Curhan G. 2007; Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 57:109–15. DOI:
10.1002/art.22466. PMID:
17266099.
29. Moon KW, Kim MJ, Choi IA, Shin K. 2022; Cardiovascular risks in Korean patients with gout: analysis using a National Health Insurance Service database. J Clin Med. 11:2124. DOI:
10.3390/jcm11082124. PMID:
35456221. PMCID:
PMC9030984.
30. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. 2004; Prevalence of the metabolic syndrome in patients with gout. J Korean Rheum Assoc. 11:349–57.
31. Jung JH, Song GG, Ji JD, Lee YH, Kim JH, Seo YH, et al. 2018; Metabolic syndrome: prevalence and risk factors in Korean gout patients. Korean J Intern Med. 33:815–22. DOI:
10.3904/kjim.2016.062. PMID:
27729624. PMCID:
PMC6030414.
32. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. 2005; The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 20:1029–33. DOI:
10.3346/jkms.2005.20.6.1029. PMID:
16361817. PMCID:
PMC2779304.
33. Cho SK, Chang Y, Kim I, Ryu S. 2018; U-shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. 70:1122–32. DOI:
10.1002/art.40472. PMID:
29694719.
34. Abraham A, Drory VE. 2014; Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: a meta-analysis. J Neurol. 261:1133–8. DOI:
10.1007/s00415-014-7331-x. PMID:
24699859.
35. Wen M, Zhou B, Chen YH, Ma ZL, Gou Y, Zhang CL, et al. 2017; Serum uric acid levels in patients with Parkinson's disease: a meta-analysis. PLoS One. 12:e0173731. DOI:
10.1371/journal.pone.0173731. PMID:
28319195. PMCID:
PMC5358777.
36. Khan AA, Quinn TJ, Hewitt J, Fan Y, Dawson J. 2016; Serum uric acid level and association with cognitive impairment and dementia: systematic review and meta-analysis. Age (Dordr). 38:16. DOI:
10.1007/s11357-016-9871-8. PMID:
26820749. PMCID:
PMC5005871.
37. Latourte A, Soumaré A, Bardin T, Perez-Ruiz F, Debette S, Richette P. 2018; Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis. 77:328–35. DOI:
10.1136/annrheumdis-2016-210767. PMID:
28754803.
39. Kim JH, Choi IA, Kim A, Kang G. 2021; Clinical association between gout and Parkinson's disease: a nationwide population-based cohort study in Korea. Medicina (Kaunas). 57:1292. DOI:
10.3390/medicina57121292. PMID:
34946237. PMCID:
PMC8704991.
41. Hu LY, Yang AC, Lee SC, You ZH, Tsai SJ, Hu CK, et al. 2020; Risk of Parkinson's disease following gout: a population-based retrospective cohort study in Taiwan. BMC Neurol. 20:338. DOI:
10.1186/s12883-020-01916-9. PMID:
32900384. PMCID:
PMC7487828.
42. Fini MA, Elias A, Johnson RJ, Wright RM. 2012; Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 1:16. DOI:
10.1186/2001-1326-1-16. PMID:
23369448. PMCID:
PMC3560981.
43. Slobodnick A, Krasnokutsky S, Lehmann RA, Keenan RT, Quach J, Francois F, et al. 2019; Colorectal cancer among gout patients undergoing colonoscopy. J Clin Rheumatol. 25:335–40. DOI:
10.1097/RHU.0000000000000893. PMID:
31764494.
44. Kuo CF, Luo SF, See LC, Chou IJ, Fang YF, Yu KH. 2012; Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine. 79:375–8. DOI:
10.1016/j.jbspin.2011.09.011. PMID:
22088929.
46. Xie Y, Xu P, Liu K, Lin S, Wang M, Tian T, et al. 2019; Hyperuricemia and gout are associated with cancer incidence and mortality: a meta-analysis based on cohort studies. J Cell Physiol. 234:14364–76. DOI:
10.1002/jcp.28138. PMID:
30693505.
47. Oh YJ, Lee YJ, Lee E, Park B, Kwon JW, Heo J, et al. 2022; Cancer risk in Korean patients with gout. Korean J Intern Med. 37:460–7. DOI:
10.3904/kjim.2020.259. PMID:
32872748. PMCID:
PMC8925955.
48. Lee JS, Myung J, Lee HA, Hong S, Lee CK, Yoo B, et al. 2021; Risk of cancer in middle-aged patients with gout: a nationwide population-based study in Korea. J Rheumatol. 48:1465–71. DOI:
10.3899/jrheum.200497. PMID:
33191287.
49. Song JU, Hwang J, Ahn JK. 2017; Serum uric acid is positively associated with pulmonary function in Korean health screening examinees. Mod Rheumatol. 27:1057–65. DOI:
10.1080/14397595.2017.1285981. PMID:
28693364.
50. Ahn JK, Hwang J, Hwang JH, Yoon WT, Chung PW, Ryu S. 2018; The association between serum uric acid and asymptomatic intracranial arterial stenosis in middle-aged Koreans. Nutr Metab Cardiovasc Dis. 28:14–22. DOI:
10.1016/j.numecd.2017.10.019. PMID:
29191476.
51. Hwang J, Hwang JH, Chung SM, Kwon MJ, Ahn JK. 2018; Association between serum uric acid and arterial stiffness in a low-risk, middle-aged, large Korean population: a cross-sectional study. Medicine (Baltimore). 97:e12086. DOI:
10.1097/MD.0000000000012086. PMID:
30200090. PMCID:
PMC6133619.
52. Hwang J, Hwang JH, Ryu S, Ahn JK. 2019; Higher serum uric acid is associated with higher lumbar spine bone mineral density in male health-screening examinees: a cross-sectional study. J Bone Miner Metab. 37:142–51. DOI:
10.1007/s00774-018-0905-4. PMID:
29372335.
53. Hwang J, Ryu S, Ahn JK. 2022; Higher levels of serum uric acid have a significant association with lower incidence of lower urinary tract symptoms in healthy Korean men. Metabolites. 12:649. DOI:
10.3390/metabo12070649. PMID:
35888773. PMCID:
PMC9322789.
54. Lee KW, Shin D. 2022; Concurrent presence of high serum uric acid and inflammation is associated with increased incidence of type 2 diabetes mellitus in Korean adult population. Sci Rep. 12:11000. DOI:
10.1038/s41598-022-15176-9. PMID:
35768559. PMCID:
PMC9243007.
55. Jeong H, Baek SY, Kim SW, Park EJ, Kim H, Lee J, et al. 2021; Gender-specific association of serum uric acid and pulmonary function: data from the Korea National Health and Nutrition Examination Survey. Medicina (Kaunas). 57:953. DOI:
10.3390/medicina57090953. PMID:
34577876. PMCID:
PMC8465554.
56. Lee YH, Song GG. 2019; Uric acid level, gout and bone mineral density: a Mendelian randomization study. Eur J Clin Invest. 49:e13156. DOI:
10.1111/eci.13156.
57. Kim K, Kang K, Sheol H, Shin J, Sim Y, Yang T, et al. 2022; The association between serum uric acid levels and 10-year cardiovascular disease risk in non-alcoholic fatty liver disease patients. Int J Environ Res Public Health. 19:1042. DOI:
10.3390/ijerph19031042. PMID:
35162067. PMCID:
PMC8834479.
58. Janssens HJ, Arts PG, Schalk BW, Biermans MC. 2017; Gout and rheumatoid arthritis, both to keep in mind in cardiovascular risk management: a primary care retrospective cohort study. Joint Bone Spine. 84:59–64. DOI:
10.1016/j.jbspin.2015.12.003. PMID:
27236260.
59. Rai SK, Burns LC, De Vera MA, Haji A, Giustini D, Choi HK. 2015; The economic burden of gout: a systematic review. Semin Arthritis Rheum. 45:75–80. DOI:
10.1016/j.semarthrit.2015.02.004. PMID:
25912932.
60. Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX. 2003; A literature review of the epidemiology and treatment of acute gout. Clin Ther. 25:1593–617. DOI:
10.1016/S0149-2918(03)80158-3. PMID:
12860487.
61. Flores NM, Nuevo J, Klein AB, Baumgartner S, Morlock R. 2019; The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 22:1–6. DOI:
10.1080/13696998.2018.1532904. PMID:
30289009.
62. Son KM, Kim JR, Park HA, Kim HA. 2022; Trends in hospital visits and healthcare costs of gout and seropositive rheumatoid arthritis in Korea from 2010 to 2017 using National Healthcare Claims. Korean J Intern Med. 37:681–90. DOI:
10.3904/kjim.2020.322. PMID:
34695882. PMCID:
PMC9082448.
63. Nielsen SM, Bartels EM, Henriksen M, Wæhrens EE, Gudbergsen H, Bliddal H, et al. 2017; Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann Rheum Dis. 76:1870–82. DOI:
10.1136/annrheumdis-2017-211472. PMID:
28866649. PMCID:
PMC5705854.
64. Zhu Y, Zhang Y, Choi HK. 2010; The serum urate-lowering impact of weight loss among men with a high cardiovascular risk profile: the Multiple Risk Factor Intervention Trial. Rheumatology (Oxford). 49:2391–9. DOI:
10.1093/rheumatology/keq256. PMID:
20805117. PMCID:
PMC2981511.
65. Dalbeth N, Chen P, White M, Gamble GD, Barratt-Boyes C, Gow PJ, et al. 2014; Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis. 73:797–802. DOI:
10.1136/annrheumdis-2013-203970. PMID:
24255548.
66. Ahn JK, Hwang J, Lee MY, Kang M, Hwang J, Koh EM, et al. 2021; How much does fat mass change affect serum uric acid levels among apparently clinically healthy Korean men? Ther Adv Musculoskelet Dis. 13:1759720X21993253. DOI:
10.1177/1759720X21993253. PMID:
33708266. PMCID:
PMC7907717.
67. Hwang J, Lee MY, Ahn JK, Cha HS. 2022; Relationship between changing body mass index and serum uric acid alteration among clinically apparently healthy Korean men. Arthritis Care Res (Hoboken). 74:1277–86. DOI:
10.1002/acr.24576. PMID:
33544980.
69. Halpern R, Mody RR, Fuldeore MJ, Patel PA, Mikuls TR. 2009; Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin. 25:1711–9. DOI:
10.1185/03007990903017966. PMID:
19485724.
70. Scheepers LEJM, van Onna M, Stehouwer CDA, Singh JA, Arts ICW, Boonen A. 2018; Medication adherence among patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum. 47:689–702. DOI:
10.1016/j.semarthrit.2017.09.007. PMID:
29198878.
71. Lee S, So MW. 2016; Adherence with urate-lowering therapies among male patients with gout in a routine clinical setting. Mod Rheumatol. 26:950–5. DOI:
10.3109/14397595.2016.1170914. PMID:
27142782.
72. Lee S, So MW, Ahn E. 2019; Long-term adherence and persistence with febuxostat among male patients with gout in a routine clinical setting. Mod Rheumatol. 29:662–8. DOI:
10.1080/14397595.2018.1483293. PMID:
29856667.
73. Chung MK, Kim SS, Cheon YH, Hong SJ, Choi HJ, Seo MR, et al. 2021; Patient perspectives and preferences regarding gout and gout management: impact on adherence. J Korean Med Sci. 36:e208. DOI:
10.3346/jkms.2021.36.e208. PMID:
34402226. PMCID:
PMC8369315.
74. Kim JW, Kwak SG, Park SH. 2018; Prescription pattern of urate-lowering therapy in Korean gout patients: data from the national health claims database. Korean J Intern Med. 33:228–9. DOI:
10.3904/kjim.2016.429. PMID:
28823114. PMCID:
PMC5768547.
75. Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. 2008; Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 59:1540–8. DOI:
10.1002/art.24209. PMID:
18975369.
76. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. 2005; Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 353:2450–61. DOI:
10.1056/NEJMoa050373. PMID:
16339094.
77. Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J, et al. 2020; Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. 396:1745–57. DOI:
10.1016/S0140-6736(20)32234-0. PMID:
33181081.
78. Pawar A, Desai RJ, Liu J, Kim E, Kim SC. 2021; Updated assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol. J Am Heart Assoc. 10:e020045. DOI:
10.1161/JAHA.120.020045. PMID:
33764153. PMCID:
PMC8174329.
79. Zhang M, Solomon DH, Desai RJ, Kang EH, Liu J, Neogi T, et al. 2018; Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population-based cohort study. Circulation. 138:1116–26. DOI:
10.1161/CIRCULATIONAHA.118.033992. PMID:
29899013. PMCID:
PMC6202230.
80. Kang EH, Choi HK, Shin A, Lee YJ, Lee EB, Song YW, et al. 2019; Comparative cardiovascular risk of allopurinol versus febuxostat in patients with gout: a nation-wide cohort study. Rheumatology (Oxford). 58:2122–9. DOI:
10.1093/rheumatology/kez189. PMID:
31098635.
81. Lee JS, Oh JS, Kim YG, Lee CK, Yoo B, Hong S. 2020; Rapid reduction in uric acid by a urate-lowering agent is associated with recurrent cardiovascular events. Med Hypotheses. 141:109740. DOI:
10.1016/j.mehy.2020.109740. PMID:
32305810.
82. Ghang BZ, Lee JS, Choi J, Kim J, Yoo B. 2022; Increased risk of cardiovascular events and death in the initial phase after discontinuation of febuxostat or allopurinol: another story of the CARES trial. RMD Open. 8:e001944. DOI:
10.1136/rmdopen-2021-001944. PMID:
35732345. PMCID:
PMC9226988.
83. Kolandaivelu K, Leiden BB, O'Gara PT, Bhatt DL. 2014; Non-adherence to cardiovascular medications. Eur Heart J. 35:3267–76. DOI:
10.1093/eurheartj/ehu364. PMID:
25265973.
84. Kang EH, Park EH, Shin A, Song JS, Kim SC. 2021; Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur Heart J. 42:4578–88. DOI:
10.1093/eurheartj/ehab619. PMID:
34508567. PMCID:
PMC8633759.
85. Eun Y, Han H, Kim K, Kang S, Lee S, Kim H, et al. 2022; Cardiovascular risk associated with allopurinol or benzbromarone treatment in patients with gout. Ther Adv Musculoskelet Dis. 14:1759720X221116409. DOI:
10.1177/1759720X221116409. PMID:
35966182. PMCID:
PMC9373176.
86. Lee KW, Oh DH, Lee C, Yang SY. 2005; Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 65:437–47. DOI:
10.1111/j.1399-0039.2005.00386.x. PMID:
15853898.
87. González-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, et al. 2015; Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 43(Database issue):D784–8. DOI:
10.1093/nar/gku1166. PMID:
25414323. PMCID:
PMC4383964.
88. Park DJ, Kang JH, Lee JW, Lee KE, Wen L, Kim TJ, et al. 2015; Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res (Hoboken). 67:280–7. DOI:
10.1002/acr.22409. PMID:
25047754.
89. Park HW, Kim DK, Kim SH, Kim S, Chae DW, Yang MS, et al. 2019; Efficacy of the HLA-B*58:01 screening test in preventing allopurinol-induced severe cutaneous adverse reactions in patients with chronic renal insufficiency-a prospective study. J Allergy Clin Immunol Pract. 7:1271–6. DOI:
10.1016/j.jaip.2018.12.012. PMID:
30580048.
90. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020; 2020 American College of Rheumatology guideline for the management of gout. Arthritis Rheumatol. 72:879–95. Erratum. DOI:
10.1002/art.41688. PMID:
33638303.
91. Ahn E, Lee S, Lee HN, Lee SG, So MW. 2019; Comparison of efficacy and safety of febuxostat in gout patients with chronic kidney disease stage 3 and stage 4/5. J Rheum Dis. 26:118–23. DOI:
10.4078/jrd.2019.26.2.118.
92. Choi SY, Choi SW, Lee S, So MW, Oh JS, Lim DH. 2021; Efficacy and tolerability of febuxostat in gout patients on dialysis. Intern Med J. 51:348–54. DOI:
10.1111/imj.14776. PMID:
32043690.
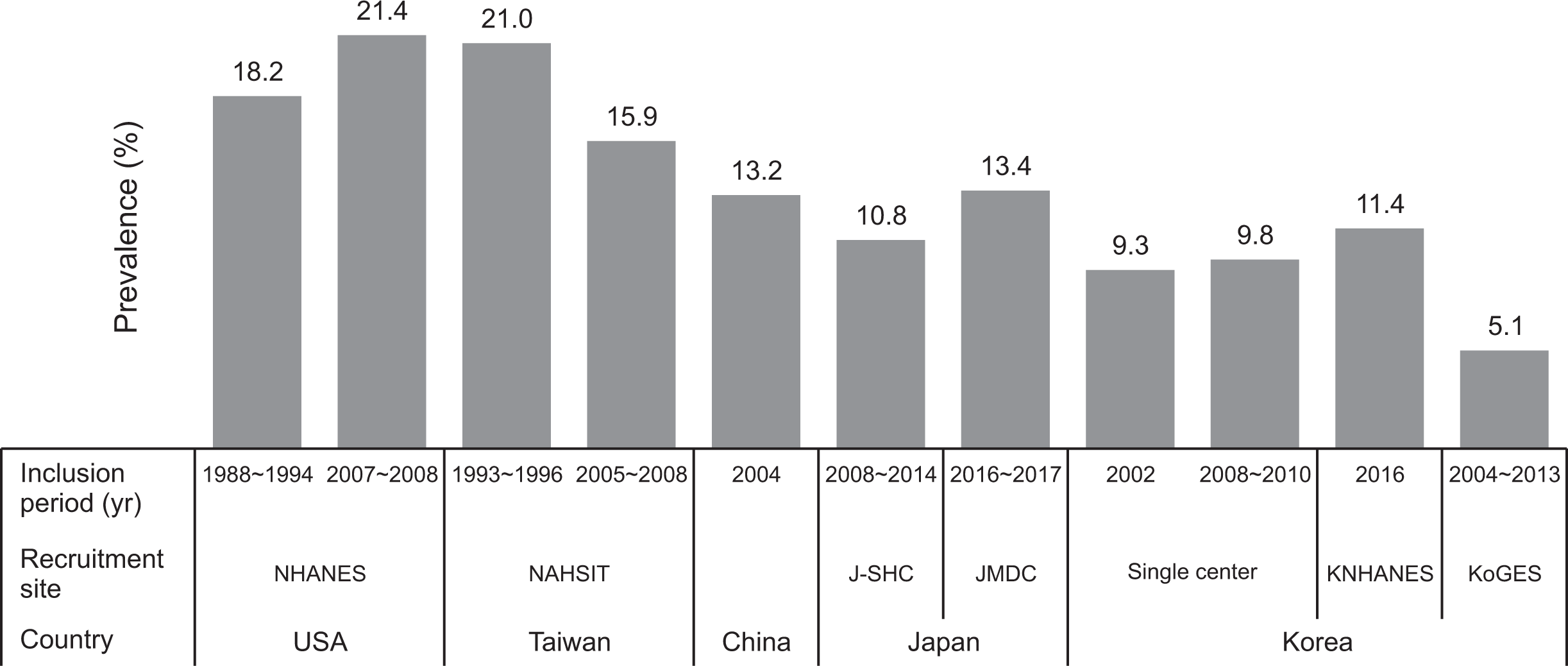
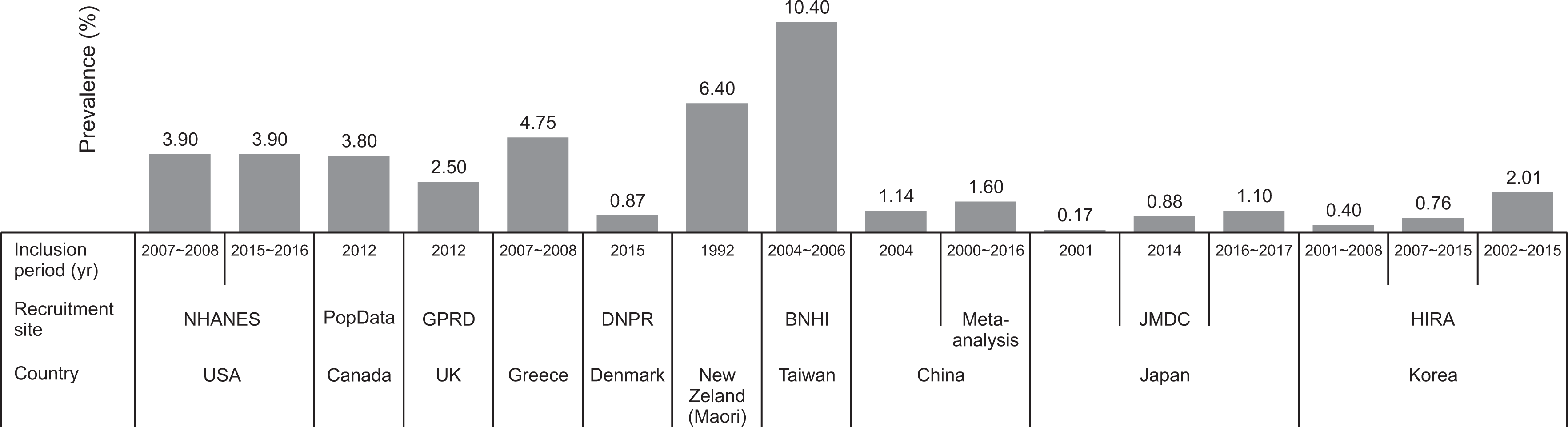




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download