Abstract
Objective
The aim of the study was to investigate the association between the levels of leptin in the circulating of individuals with rheumatoid arthritis (RA) and the severity of the disease.
Methods
We looked through the databases of Embase, Medline, and the Cochrane Library. We conducted a meta-analysis on the correlations between circulating leptin and the Disease Activity Score 28-erythrocyte sedimentation rate (DAS28-ESR) and C-reactive protein (CRP) levels in RA patients, as well as a meta-analysis of circulating or circulating leptin levels in RA patients.
Results
This meta-analysis study analyzed 42 different comparisons from 37 different publications, including a total of 2,350 patients with RA and 1,815 controls. The RA group had substantially higher leptin levels than the control group (standardized mean difference [SMD]=0.507, 95% confidence interval [CI]=0.309~0.704, p<0.001). The finding that RA patients had higher leptin levels was unaffected by sample size. The correlation between circulating leptin levels and DAS28 is statistically significant (correlation coefficient=0.247, 95% CI=0.087~0.396, p=0.003). Leptin levels are also correlated with CRP levels (correlation coefficient=0.203, 95% CI=0.048~0.349, p=0.010).
Conclusion
This comprehensive meta-analysis demonstrates that the circulating leptin levels of RA patients are elevated, and provides compelling evidence of the significant relationship between leptin levels and the activity of RA. The findings of this research suggest that leptin plays a significant role in the pathophysiology of this disease.
Rheumatoid arthritis (RA) is a persistent and debilitating autoimmune disease that affects the joints and causes inflammation and damage to the surrounding tissue. The disease is initiated by the invasion of the synovium by various immune system cells, including T cells, B cells, macrophages, dendritic cells, and neutrophils. This leads to a chronic state of inflammation that can have a significant impact on the individual’s quality of life and life expectancy.
Leptin, a hormone produced primarily by white adipose tissue cells, was initially believed to play a role in regulating hunger and energy levels. However, recent research has revealed that leptin has a much broader role in the regulation of inflammation. Leptin and its receptors are similar in structure and function to cytokines of the IL-6 family and play a role in mediating the immune response. Leptin has both pro-inflammatory and anti-inflammatory effects, and it can activate the Th1 phenotype of T cells, leading to the production of pro-inflammatory cytokines such as TNF-[Symbol - a] and IL-6. However, it can also increase the production of anti-inflammatory molecules, such as IL-1 receptor antagonists and IL-4.
Leptin levels have been found to increase in conditions that cause inflammation and play a role in the regulation of the immune response in autoimmune diseases. Despite the potential importance of leptin in RA, the relationship between leptin levels and disease activity has produced conflicting results. Some studies have shown a positive relationship between leptin levels and RA, while others have shown no relationship or even a negative correlation. This discrepancy in the literature may be due to small sample sizes, low statistical power, clinical heterogeneity, or leptin gene expression owing to the energy or fat status [1]. The purpose of this research is to examine the relationship between circulating leptin levels and RA patients using a meta-analytic approach.
We searched for studies that compared circulating leptin levels between RA patients and healthy controls, or that examined the relationship between leptin levels and RA disease activity. Publications were discovered by searching Medline (from 1971), Embase (from 1946), and Cochrane databases (from 1993) (up to December 2022). The terms, “rheumatoid arthritis” and “leptin” were used for the literature search. References of available articles were thoroughly investigated in order to discover research that the aforementioned key phrases had missed. To be included, studies had to be case-control or cohort studies, and give leptin levels in both RA and controls, or provide pertinent information on the relationship between circulating leptin levels and RA activity, as defined by Disease Activity Score 28-erythrocyte sedimentation rate (DAS28-ESR) or C-reactive protein (CRP) level. Each study’s authors, publication years, ethnicities, numbers, means and standard deviations, and correlation coefficients were gathered. Using the Newcastle–Ottawa scale, we evaluated the overall quality of the meta-analysis studies. Six to nine points imply high methodological quality, with nine being the highest possible score.
We gathered data from studies to look for associations between leptin and RA, as well as to determine if leptin is linked to DAS28 or CRP. Results were consistently presented as standard deviations of the mean and 95% confidence intervals (Cis). Using Cochran’s Q-statistics, we examined both within- and between-trial heterogeneity and variance [2]. If the Q-statistic indicated trial heterogeneity, the random-effects model was used (p<0.10). Elsewhere, a fixed-effects model was used. For statistical analysis, a comprehensive meta-analysis was used (Biostat, Englewood, NJ, USA). p-values less than 0.05 were regarded statistically significant.
In a meta-regression analysis, ethnicity, publication year, and sample size were used to better comprehend the observed heterogeneity in the meta-analysis. We ran a sensitivity analysis to see how the pooled standardized mean difference (SMD) would change if individual trials were omitted. We used the “trim and fill” procedure to address the publication bias in the summary estimates.
We discovered 37 articles for this meta-analysis [3-39] (Figure 1). Five of the studies reported data pertaining to two distinct groups. For the meta-analysis, 2,350 RA patients and 1,815 controls were selected from 42 comparisons (Table 1). The quality of research was similarly high across the board (all studies obtained scores between 6 and 8). Table 1 contains the demographic information for each participant.
The RA group had substantially higher leptin levels than the control group (SMD=0.507, 95% CI=0.309~0.704, p<0.001) (Table 2, Figure 2). Leptin levels in the RA group were also higher in Caucasians, Arabs, and Hispanics (SMD=0.418, 95% CI=0.145~0.692, p=0.003; SMD=1.317, 95% CI=0.554~2.081, p=0.001; SMD=0.652, 95% CI=0.317~0.988, p=0.001, respectively) (Table 2, Figure 2). The fact that RA patients had higher leptin levels was unaffected by sample size (Table 2).
A meta-analysis of leptin levels in RA patients revealed heterogeneity across studies (Table 2). In a meta-analysis of leptin levels in RA patients, ethnicity and sample size did not impact heterogeneity, but publication year was a significant predictor. As the publication year becomes more recent, there was a decrease in the heterogeneity of the study’s findings, indicating a trend towards greater consistency and reliability in research over time. A sensitivity analysis found that the omission of a single study did not significantly impact the meta-findings analyses. The funnel plot was asymmetrical, thus, the “trim and fill” method was used to correct this (Figure 4). In spite of the modification, the substantial SMD (SMD=0.774, 95% CI=0.565~0.975) remained unaltered.
Leptin regulation is crucial for maintaining optimal immunological and neuroendocrine function. As previously documented, leptin has the ability to suppress the development of T-cells into the Th1 phenotype, activate monocytes/macrophages and stimulate the production of proinflammatory cytokines. Moreover, it has been observed that proinflammatory cytokines can increase the levels of leptin in circulation. Leptin plays a significant role in T-cell-mediated inflammation by regulating T-helper cell activity. This regulation results in the induction of macrophages and granulocytes, two cell types that contribute to lymphopoiesis and myelopoiesis, leading to an increase in phagocytosis and the production of proinflammatory cytokines. Interactions between the immune system and neuroendocrine system may also contribute to the development of RA. However, the specific role of leptin in chronic inflammatory diseases such as RA is still uncertain.
This meta-analysis showed that the RA group had significantly higher leptin levels in the circulating compared to the control group. The study also discovered a connection between leptin levels and RA activity using the DAS28 and CRP. It was noted that the clinical improvement of individuals with RA who fasted was associated with a decrease in circulating leptin levels and a transition to Th2 cytokine production, further emphasizing the impact of adipocytokines on the proinflammatory state in RA. These findings suggest that leptin might have the ability to induce a Th1 response and change the inflammatory process in RA. Leptin levels in the RA group were not statistically differentto those seen in Asians. There are several possible reasons for the lack of noticeable statistical differences in leptin levels between the RA and control groups among individuals of Asian descent. These could include the impact of confounding variables, the heterogeneity within the RA group, and the diversity of ethnic backgrounds among the participants. Furthermore, genetic and environmental factors may also have contributed to the observed levels of leptin.
However, several issues were identified in this meta-analysis that need to be addressed. Firstly, there is a lack of large-scale studies examining the relationship between leptin levels and RA activity. Secondly, the variety of publications evaluated might have influenced the results, and factors such as body mass index (BMI), sex, and a lack of clinical data were not specifically assessed. It has been found that circulating leptin levels were highly correlated with BMI, with the exception of the BMI range near the bottom of the U shape [40]. Despite conducting a sensitivity test and meta-regression analysis, there was not enough data to draw definitive conclusions. Thirdly, while we did ethnicity-based analyses to investigate possible variations in the association between leptin levels and RA activity across various ethnic groups, we acknowledge that a specific area does not necessarily reflect a particular ethnicity. Despite these limitations, the meta-analysis provides the most comprehensive data to date on leptin status in RA patients, and the results suggest that leptin may play a role in the development and progression of RA.
In conclusion, this comprehensive meta-analysis, which incorporates data from 37 separate studies and a total of 2,350 RA patients and 1,815 controls demonstrates that the circulating leptin levels of RA patients are elevated, and provides compelling evidence of the significant relationship between leptin levels and the activity of RA. The findings of this research suggesting that leptin plays a significant role in the pathophysiology of this disease. Further large-scale studies are needed to establish the exact role of leptin in RA and to determine if targeting leptin levels can be a potential therapeutic strategy for the treatment of RA.
Notes
CONFLICT OF INTEREST
Y.H.L. has been an editorial board member since March 2003, but has no role in the decision to publish this article. G.G.S. has no conflict of interest.
AUTHOR CONTRIBUTIONS
Y.H.L. was involved in conception and design of study, acquisition of data, analysis and/or interpretation of data, drafting the manuscript, revising the manuscript critically for important intellectual content. G.G.S. was involved in conception and design of study, analysis and interpretation of data, drafting the manuscript.
REFERENCES
1. Münzberg H, Heymsfield SB. 2019; New insights into the regulation of leptin gene expression. Cell Metab. 29:1013–4. DOI: 10.1016/j.cmet.2019.04.005. PMID: 31067443. PMCID: PMC7346278.

2. Egger M, Smith GD, Phillips AN. 1997; Meta-analysis: principles and procedures. BMJ. 315:1533–7. DOI: 10.1136/bmj.315.7121.1533. PMID: 9432252. PMCID: PMC2127925.

3. Magali Chamorro-Melo Y, Calixto OJ, Bello-Gualtero JM, Bautista-Molano W, Beltran-Ostos A, Romero-Sánchez C. 2022; Evaluation of the adipokine profile (adiponectin, resistin, adipsin, vaspin, and leptin) in patients with early rheumatoid arthritis and its correlation with disease activity. Reumatologia. 60:192–9. DOI: 10.5114/reum.2022.117839. PMID: 35875721. PMCID: PMC9301668.

4. Cheleschi S, Tenti S, Bedogni G, Fioravanti A. 2022; Circulating Mir-140 and leptin improve the accuracy of the differential diagnosis between psoriatic arthritis and rheumatoid arthritis: a case-control study. Transl Res. 239:18–34. DOI: 10.1016/j.trsl.2021.08.001. PMID: 34380068.

5. Zhang Y, Johansson L, Andersson-Assarsson J, Taube M, Peltonen M, Svensson PA, et al. 2021; Adiponectin associates with rheumatoid arthritis risk in overweight and obesity independently of other adipokines. J Clin Med. 10:2791. DOI: 10.3390/jcm10132791. PMID: 34201946. PMCID: PMC8267689.

6. Turgunova LG, Shalygina AA, Zalkalns JP, Klyuyev DA, Akhmaltdinova LL, Dosmagambetova RS. 2021; Assessment of adipokines, CXCL16 chemokine levels in patients with rheumatoid arthritis combined with metabolic syndrome. Clin Med Insights Arthritis Musculoskelet Disord. 14:1179544120985860. DOI: 10.1177/1179544120985860. PMID: 33613035. PMCID: PMC7868477.

7. Chen YM, Chen PK, Chang CK, Lin CC, Chen HH, Lan JL, et al. 2020; Association of apolipoprotein E polymorphism with adipokines and cardiovascular disease risk in rheumatoid arthritis patients. Life (Basel). 10:330. DOI: 10.3390/life10120330. PMID: 33297350. PMCID: PMC7762228.

8. Tao SS, Dan YL, Wu GC, Zhang Q, Zhang TP, Fan YG, et al. 2020; Association of leptin gene polymorphisms with rheumatoid arthritis in a Chinese population. Biomed Res Int. 2020:3789319. DOI: 10.1155/2020/3789319. PMID: 33083462. PMCID: PMC7559230.
9. Chihara K, Hattori N, Ichikawa N, Matsuda T, Saito T. 2020; Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis. Sci Rep. 10:15932. DOI: 10.1038/s41598-020-73068-2. PMID: 32985609. PMCID: PMC7522978.

10. Hoffman E, Rahat MA, Feld J, Elias M, Rosner I, Kaly L, et al. 2019; Effects of tocilizumab, an anti-interleukin-6 receptor antibody, on serum lipid and adipokine levels in patients with rheumatoid arthritis. Int J Mol Sci. 20:4633. DOI: 10.3390/ijms20184633. PMID: 31540528. PMCID: PMC6770905.

11. Dervišević A, Resić H, Sokolović Š, Babić N, Avdagić N, Začiragić A, et al. 2018; Leptin is associated with disease activity but not with anthropometric indices in rheumatoid arthritis patients. Arch Med Sci. 14:1080–6. DOI: 10.5114/aoms.2017.65080. PMID: 30154891. PMCID: PMC6111354.

12. Angel-Chávez LI, Ruelas-Cinco E, Hernández-Bello J, Castro E, Vázquez-Villamar M, Parra-Rojas I, et al. 2018; Influence of serum leptin levels and Q223R leptin receptor polymorphism on clinical characteristic of patients with rheumatoid arthritis from Western Mexico. EJIFCC. 29:26–35.
13. Rodríguez-Carrio J, Alperi-López M, López P, López-Mejías R, Alonso-Castro S, Abal F, et al. 2017; High triglycerides and low high-density lipoprotein cholesterol lipid profile in rheumatoid arthritis: a potential link among inflammation, oxidative status, and dysfunctional high-density lipoprotein. J Clin Lipidol. 11:1043–54.e2. DOI: 10.1016/j.jacl.2017.05.009. PMID: 28662934.
14. Najafizadeh SR, Farahmand G, Roudsari AT, Heidari B, Larry M, Nargesi AA, et al. 2016; Absence of a positive correlation between CRP and leptin in rheumatoid arthritis. Heliyon. 2:e00205. DOI: 10.1016/j.heliyon.2016.e00205. PMID: 27981248. PMCID: PMC5148782.

15. Gülkesen A, Akgöl G, Tuncer T, Kal GA, Telo S, Poyraz AK, et al. 2016; Relationship between leptin and neopterin levels and disease activation parameters in patients with rheumatoid arthritis. Arch Rheumatol. 31:333–9. DOI: 10.5606/ArchRheumatol.2016.5893. PMID: 30375574. PMCID: PMC6190973.

16. Gómez-Bañuelos E, Navarro-Hernández RE, Corona-Meraz F, Madrigal-Ruíz PM, Martín-Marquez BT, Pizano-Martinez OE, et al. 2015; Serum leptin and serum leptin/serum leptin receptor ratio imbalance in obese rheumatoid arthritis patients positive for anti-cyclic citrullinated peptide antibodies. Arthritis Res Ther. 17:335. DOI: 10.1186/s13075-015-0850-8. PMID: 26589684. PMCID: PMC4654826.

17. Oner SY, Volkan O, Oner C, Mengi A, Direskeneli H, Tasan DA. 2015; Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol Port. 40:50–4.
18. Abdalla M, Effat D, Sheta M, Hamed WE. 2014; Serum leptin levels in rheumatoid arthritis and relationship with disease activity. Egypt Rheumatol. 36:1–5. DOI: 10.1016/j.ejr.2013.10.002.

19. Toussirot E, Grandclément E, Gaugler B, Michel F, Wendling D, Saas P, et al. 2013; Serum adipokines and adipose tissue distribution in rheumatoid arthritis and ankylosing spondylitis. A comparative study. Front Immunol. 4:453. DOI: 10.3389/fimmu.2013.00453. PMID: 24379815. PMCID: PMC3861781.

20. Olama SM, Senna MK, Elarman M. 2012; Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int. 32:683–90. DOI: 10.1007/s00296-010-1698-5. PMID: 21140264.

21. Allam A, Radwan A. 2012; The relationship of serum leptin levels with disease activity in Egyptian patients with rheumatoid arthritis. Egypt Rheumatol. 34:185–90. DOI: 10.1016/j.ejr.2012.08.001.

22. Kopec-Medrek M, Kotulska A, Widuchowska M, Adamczak M, Więcek A, Kucharz EJ. 2012; Plasma leptin and neuropeptide Y concentrations in patients with rheumatoid arthritis treated with infliximab, a TNF-α antagonist. Rheumatol Int. 32:3383–9. DOI: 10.1007/s00296-011-2182-6. PMID: 22048440. PMCID: PMC3505553.

23. Yoshino T, Kusunoki N, Tanaka N, Kaneko K, Kusunoki Y, Endo H, et al. 2011; Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern Med. 50:269–75. DOI: 10.2169/internalmedicine.50.4306. PMID: 21325757.

24. Ismail F, Ali HA, Ibrahim HM. 2011; Possible role of leptin, and tumor necrosis factor-alpha in hypoandrogenicity in patients with early rheumatoid arthritis. Egypt Rheumatol. 33:209–15. DOI: 10.1016/j.ejr.2011.07.006.

25. El-Batch MM, Zakaria SS, Farouk G, El Saadany H, Selim M. 2010; Changes in visfatin, adiponectin, leptin and ghrelin levels in patients with rheumatoid arthritis and their correlation with disease activity. Turk Biyokim Derg. 35:50–7.
26. Seven A, Güzel S, Aslan M, Hamuryudan V. 2009; Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol Int. 29:743–7. DOI: 10.1007/s00296-008-0764-8. PMID: 19009296.
27. Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. 2009; Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 60:1906–14. DOI: 10.1002/art.24626. PMID: 19565493. PMCID: PMC2894567.

28. Canoruç N, Kale E, Turhanoğlu AD, Özmen Ş, Ogün C, Kaplan A. DOI: 10.3906/sag-0801-13.
29. Hizmetli S, Kisa M, Gokalp N, Bakici MZ. 2007; Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis? Rheumatol Int. 27:335–8. DOI: 10.1007/s00296-006-0264-7. PMID: 17102942.

30. Gunaydin R, Kaya T, Atay A, Olmez N, Hur A, Koseoglu M. 2006; Serum leptin levels in rheumatoid arthritis and relationship with disease activity. South Med J. 99:1078–83. DOI: 10.1097/01.smj.0000240625.27772.79. PMID: 17100028.
31. Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gómez-Reino JJ, et al. 2006; Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 65:1198–201. DOI: 10.1136/ard.2005.046540. PMID: 16414972. PMCID: PMC1798289.

32. Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW. 2005; Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis. 64:1195–8. DOI: 10.1136/ard.2004.032243. PMID: 15731289. PMCID: PMC1755600.

33. Toussirot E, Nguyen NU, Dumoulin G, Aubin F, Cédoz JP, Wendling D. 2005; Relationship between growth hormone-IGF-I-IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology (Oxford). 44:120–5. DOI: 10.1093/rheumatology/keh421. PMID: 15466894.

34. Härle P, Pongratz G, Weidler C, Büttner R, Schölmerich J, Straub RH. 2004; Possible role of leptin in hypoandrogenicity in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 63:809–16. DOI: 10.1136/ard.2003.011619. PMID: 15194576. PMCID: PMC1755074.
35. Bokarewa M, Bokarew D, Hultgren O, Tarkowski A. 2003; Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis. 62:952–6. DOI: 10.1136/ard.62.10.952. PMID: 12972473. PMCID: PMC1754314.
36. Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K. 2002; [Serum leptin levels in patients with rheumatoid arthritis are correlated with body mass index]. Rinsho Byori. 50:524–7. Japanese.
37. Tokarczyk-Knapik A, Nowicki M, Wyroślak J. 2002; [The relation between plasma leptin concentration and body fat mass in patients with rheumatoid arthritis]. Pol Arch Med Wewn. 108:761–7. Polish.
38. Salazar-Páramo M, González-Ortiz M, González-López L, Sánchez-Ortiz A, Valera-González IC, Martínez-Abundis E, et al. 2001; Serum leptin levels in patients with rheumatoid arthritis. J Clin Rheumatol. 7:57–9. DOI: 10.1097/00124743-200102000-00016. PMID: 17039093.
39. Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M. 1999; Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 48:745–8. DOI: 10.1016/S0026-0495(99)90174-9. PMID: 10381149.

40. Cheng J, Luo Y, Li Y, Zhang F, Zhang X, Zhou X, et al. 2022; Sex- and body mass index-specific reference intervals for serum leptin: a population based study in China. Nutr Metab (Lond). 19:54. DOI: 10.1186/s12986-022-00689-x. PMID: 35941672. PMCID: PMC9358897.
Fig. 1
The procedure for selecting the studies included in this meta-analysis. RA: rheumatoid arthritis.
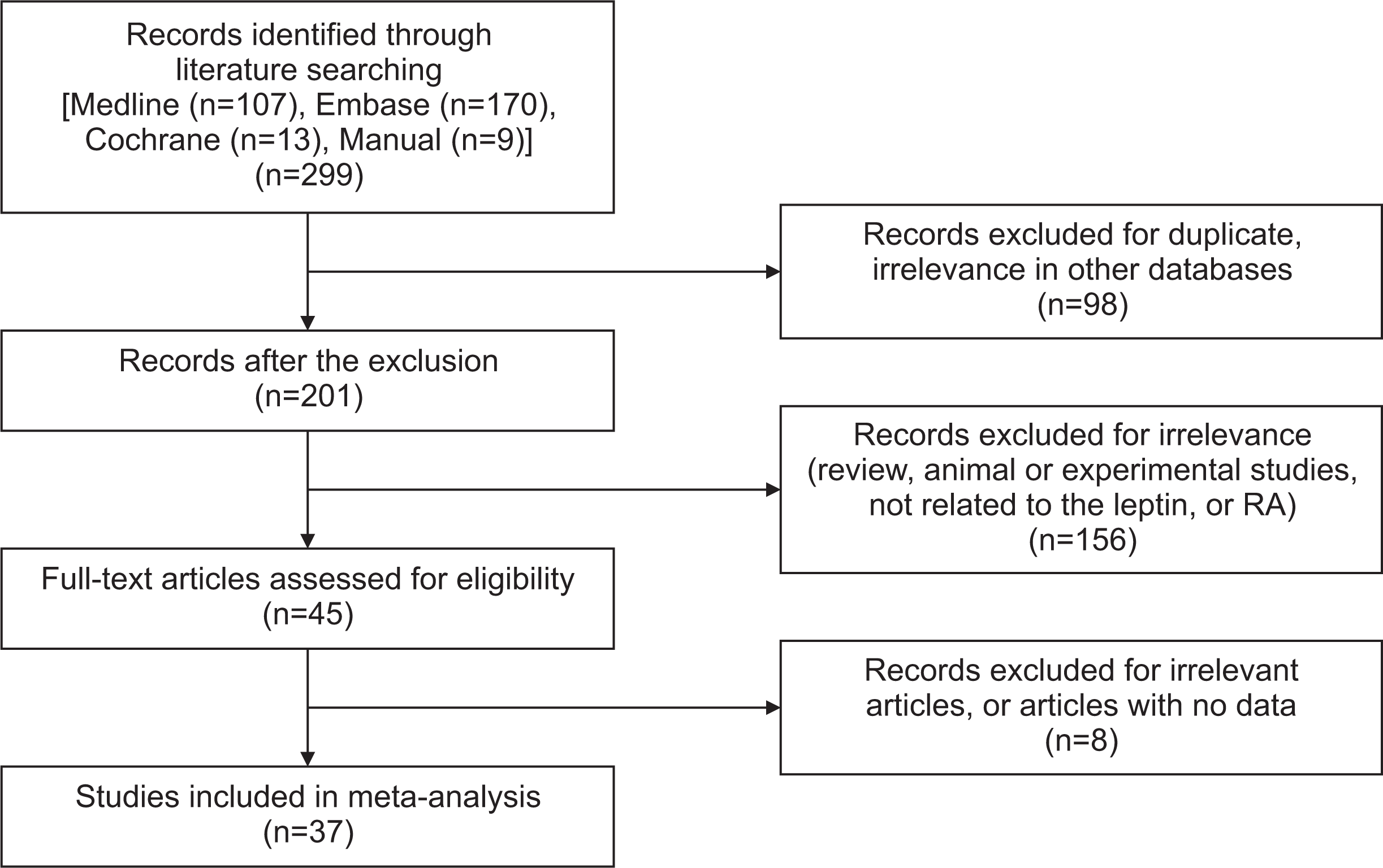
Fig. 2
Meta-analysis investigating the relationship between leptin and RA, taking into account all study participants (A) and considering any racial groups (B). CI: confidence interval, RA: rheumatoid arthritis.
Fig. 3
Meta-analysis of the relationship between leptin and the DAS28-ESR (A) as well as CRP (B). CI: confidence interval, DAS28-ESR: Disease Activity Score 28-erythrocyte sedimentation rate, CRP: C-reactive protein.
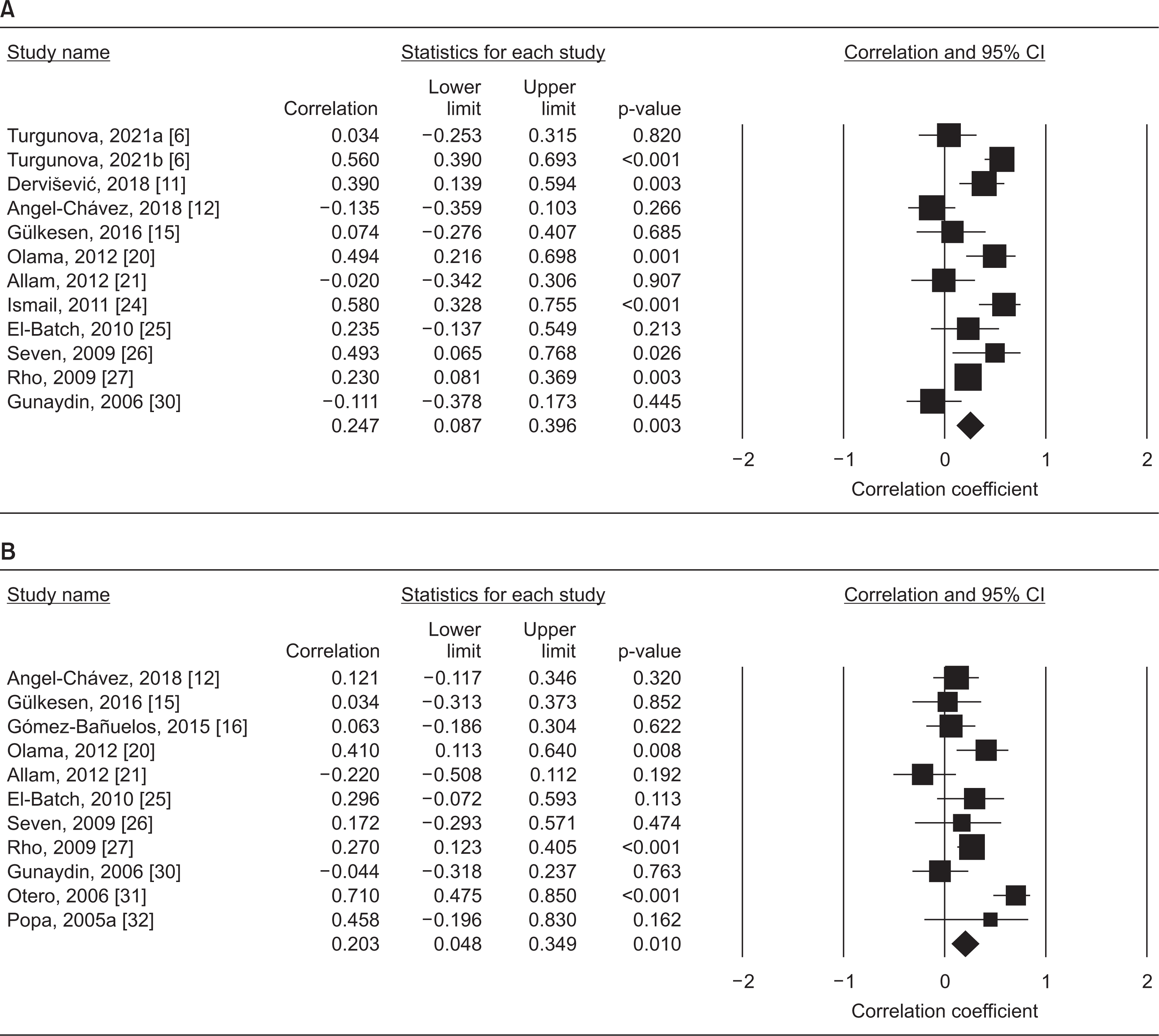
Fig. 4
The funnel plot displaying the results of the studies that investigated the connection between RA and leptin levels. The filled circles indicate the studies that demonstrate publication bias. The diamonds at the bottom of the graph represent the summary estimates of the impact, both before and after accounting for publication bias. RA: rheumatoid arthritis.
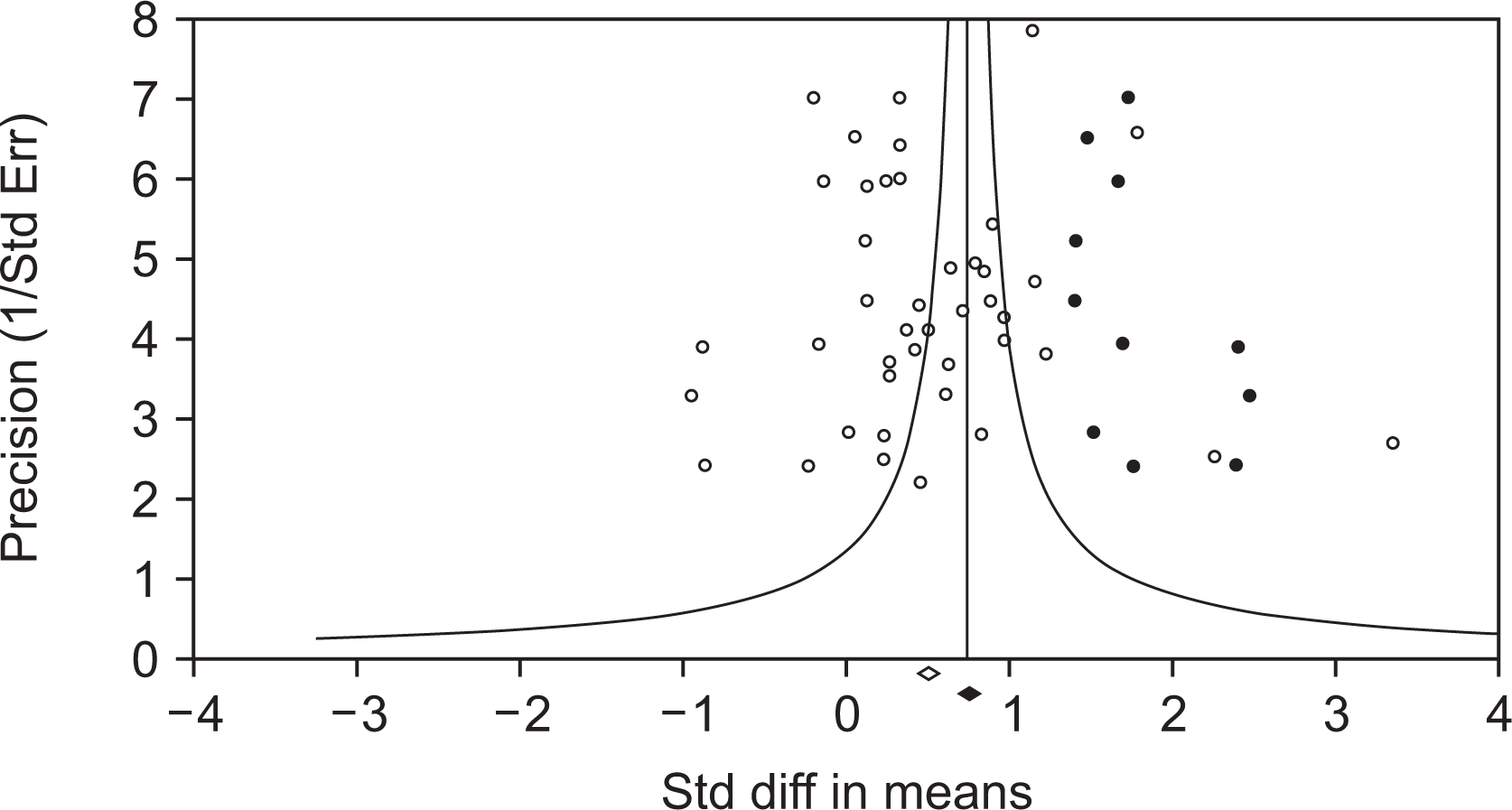
Table 1
Individual study characteristics analyzed in the meta-analysis
| Reference | Country | Ethnicity | Number | Result | ||||
|---|---|---|---|---|---|---|---|---|
| Case | Control | SMD | Magnitude* | p-value | ||||
| Magali Chamorro-Melo, 2022 [3] | Colombia | Hispanic | 51 | 51 | 0.853 | Large | <0.001 | |
| Cheleschi, 2022 [4] | UK | Caucasian | 50 | 50 | 0.643 | Large | 0.002 | |
| Zhang. 2021 [5] | Sweden | Caucasian | 82 | 88 | 0.055 | Small | 0.718 | |
| Turgunova, 2021a [6] | Kazaklstan | Asian | 48 | 63 | 0.126 | Small | 0.512 | |
| Turgunova, 2021b [6] | Kazaklstan | Asian | 82 | 63 | −0.131 | Small | 0.435 | |
| Chen, 2020 [7] | China | Asian | 150 | 48 | 0.336 | Medium | 0.044 | |
| Tao, 2020 [8] | China | Asian | 103 | 95 | −0.197 | Small | 0.168 | |
| Chihara, 2020 [9] | Japan | Asian | 136 | 78 | 0.331 | Medium | 0.021 | |
| Hoffman, 2019 [10] | Israel | Caucasian | 40 | 40 | 0.134 | Small | 0.550 | |
| Dervišević, 2018 [11] | Bosnia | Caucasian | 55 | 25 | 0.374 | Medium | 0.123 | |
| Angel-Chávez, 2018 [12] | Mexico | Hispanic | 70 | 74 | 0.251 | Medium | 0.134 | |
| Rodríguez-Carrio, 2017a [13] | Spain | Caucasian | 84 | 83 | 0.333 | Medium | 0.033 | |
| Rodríguez-Carrio, 2017b [13] | Spain | Caucasian | 29 | 83 | 0.889 | Large | <0.001 | |
| Najafizadeh, 2016 [14] | Iran | Arab | 75 | 40 | 0.794 | Large | <0.001 | |
| Gülkesen, 2016 [15] | Turkey | Caucasian | 33 | 24 | 0.272 | Medium | 0.312 | |
| Gómez-Bañuelos, 2015 [16] | Mexico | Hispanic | 64 | 66 | 0.905 | Large | <0.001 | |
| Oner, 2015 [17] | Turkey | Caucasian | 106 | 52 | 0.136 | Small | 0.421 | |
| Abdalla, 2014 [18] | Egypt | Arab | 60 | 30 | 0.454 | Medium | 0.045 | |
| Toussirot, 2013 [19] | France | Caucasian | 30 | 31 | 0.426 | Medium | 0.100 | |
| Olama, 2012 [20] | Egypt | Arab | 40 | 30 | 1.228 | Large | <0.001 | |
| Allam, 2012 [21] | Egypt | Arab | 37 | 34 | 0.973 | Large | <0.001 | |
| Kopec-Medrek, 2012 [22] | Poland | Caucasian | 16 | 16 | 0.020 | Small | 0.955 | |
| Yoshino, 2011 [23] | Japan | Asian | 141 | 146 | 1.146 | Large | <0.001 | |
| Ismail, 2011 [24] | Egypt | Arab | 40 | 30 | 3.355 | Large | <0.001 | |
| El-Batch, 2010 [25] | Turkey | Caucasian | 30 | 15 | 2.263 | Large | <0.001 | |
| Seven, 2009 [26] | Turkey | Caucasian | 20 | 15 | 0.836 | Large | 0.019 | |
| Rho, 2009 [27] | USA | Caucasian | 167 | 91 | 1.791 | Large | <0.001 | |
| Canoruç, 2009 [28] | Turkey | Caucasian | 52 | 52 | 1.163 | Large | <0.001 | |
| Hizmetli, 2007 [29] | Turkey | Caucasian | 41 | 25 | −0.165 | Small | 0.517 | |
| Gunaydin, 2006 [30] | Turkey | Caucasian | 50 | 34 | 0.967 | Large | <0.001 | |
| Otero, 2006 [31] | Spain | Caucasian | 31 | 18 | 0.618 | Large | 0.041 | |
| Popa, 2005a [32] | Netherlands | Caucasian | 11 | 9 | 0.461 | Medium | 0.311 | |
| Popa, 2005b [32] | Netherlands | Caucasian | 20 | 9 | 0.236 | Medium | 0.558 | |
| Toussirot, 2005 [33] | France | Caucasian | 38 | 32 | 0.512 | Large | 0.036 | |
| Härle, 2004a [34] | Greece | Caucasian | 8 | 28 | −0.854 | Large | 0.039 | |
| Härle, 2004b [34] | Greece | Caucasian | 22 | 26 | −0.937 | Large | 0.002 | |
| Bokarewa, 2003 [35] | Sweden | Caucasian | 49 | 34 | 0.720 | Large | 0.002 | |
| Nishiya, 2002a [36] | Japan | Asian | 14 | 10 | −0.224 | Medium | 0.589 | |
| Nishiya, 2002b [36] | Japan | Asian | 35 | 10 | 0.238 | Medium | 0.509 | |
| Tokarczyk-Knapik, 2002 [37] | Poland | Caucasian | 52 | 24 | −0.869 | Large | 0.001 | |
| Salazar-Páramo, 2001 [38] | Mexico | Hispanic | 30 | 27 | 0.634 | Large | 0.020 | |
| Anders, 1999 [39] | Greece | Caucasian | 58 | 16 | 0.273 | Medium | 0.335 | |
Table 2
Meta-analysis of the relationship between circulating leptin levels and rheumatoid arthritis
Table 3
Meta-analysis of the correlation between leptin levels and RA activity (DAS28, CRP)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download