Abstract
Background
In patients with suspected pulmonary embolism (PE), the literature suggests the overuse of computerized tomography pulmonary angiography (CTPA) and underuse of clinical decision rules before imaging request. This study determined the potential for avoidable CTPA using the modified Wells score (mWS) and D-dimer assay in patients with suspected PE.
Methods
This hospital-based retrospective study analyzed the clinical data of 661 consecutive patients with suspected PE who underwent CTPA in the emergency department of a tertiary hospital for the use of a clinical prediction rule (mWS) and D-dimer assay. The score was calculated retrospectively from the available data in the files of patients who did not have a documented clinical prediction rule. Overuse (avoidable) CTPA was defined as D-dimer negativity and PE unlikely for this study.
Results
Of 661 patients’ data examined, clinical prediction rules were documented in 15 (2.3%). In total, 422 patients (63.8%) had required information on modified Wells criteria and D-dimer assays and were included for further analysis. PE on CTPA was present in 22 (5.21%) of PE unlikely (mWS ≤4) and 1 (0.24%) of D-dimer negative patients. Thirty patients (7.11%) met the avoidable CTPA (DD negative+PE unlikely) criteria, and it was significantly associated with dyspnea. The value of sensitivity of avoidable CTPA was 100%, whereas the positive predictive value was 90.3%.
Pulmonary embolism (PE) is estimated to occur in approximately 70 cases per 100,000 [1, 2]. However, the nationwide prevalence of PE in Saudi Arabia remains unknown. A single tertiary care center study suggested that the annual incidence rate of the first venous thromboembolism was 1.7 per 1,000 patients [3]. Other hospital-based studies reported a range of 5–33.8 [4-7]. Computed tomography pulmonary angiography (CTPA) has been the most frequently used method for diagnosing PE, with a sensitivity and specificity of 83% and 96%, respectively [8]; however, it may be accompanied by the risk of side effects associated with using contrast media [9].
The use of CTPA has increased significantly over the last decade, with several recent studies highlighting its overuse in emergency departments [10-12], and it does not appear to contribute to population-level mortality reduction [8]. CTPA may have a low yield of PE, and even those with positive results have small subsegmental emboli that may be clinically insignificant [13, 14].
A large study that examined the appropriateness of CTPA use in patients in the emergency department revealed that a substantial proportion of CTPA use could have been avoided [15, 16]. The overuse of such investigations may burden patients and the healthcare system [17-19].
Important issues arise for clinicians in this situation, including selecting a patient for CTPA if a patient is suspected of having PE to avoid unnecessary investigation. To address this issue, Wells et al. [20, 21] proposed a clinical prediction rule and D-dimer testing as an algorithm for evaluating patients with suspected PE. An algorithm incorporating the Wells score and D-dimer testing in large-sample studies demonstrated a high negative predictive value [21, 22]. Later modifications to the Wells score defined individuals with a Wells score of ≤4 as PE unlikely and recommended D-dimer testing. A normal D-dimer level does not necessitate imaging, whereas a higher D-dimer level does [15]. The European Society of Cardiology recommends that this algorithm be used in the clinical setting [23].
There are reports of decreased imaging requests when a clinical prediction rule is used in conjunction with the D-dimer assay [24-26]. Pasha et al. [25] found that using a pre-test clinical prediction rule in conjunction with the D-dimer assay had a negative predictive value of 99.7%, and the risk of mortality associated with PE was reduced to <1%.
The extent to which pre-test clinical prediction rules and D-dimer assays are used in routine hospital practice, as well as their utility in determining the prescription for CTPA, is unknown, although indirect evidence suggests underutilization [25]. Thus, it is likely that low-risk patients would be subjected to avoidable CTPA and its associated adverse effects, such as radiation, increased expenditure, contrast-induced complications, and increased length of stay in the emergency department [26, 27].
Few studies have evaluated the diagnostic efficacy of pretest clinical prediction rules and D-dimer assay before prescribing CTPA in Saudi Arabian tertiary care settings. Additionally, Owaidah et al. [28] reported that the prediction rule might help predict PE, and a significant portion of the requested imaging could have been [16, 29]. Thus, this study was conducted to determine whether a clinical prediction rule combined with D-dimer assay can accurately predict the proportion of avoidable CTPA in unselected patients with suspected PE who underwent CTPA in a tertiary care center.
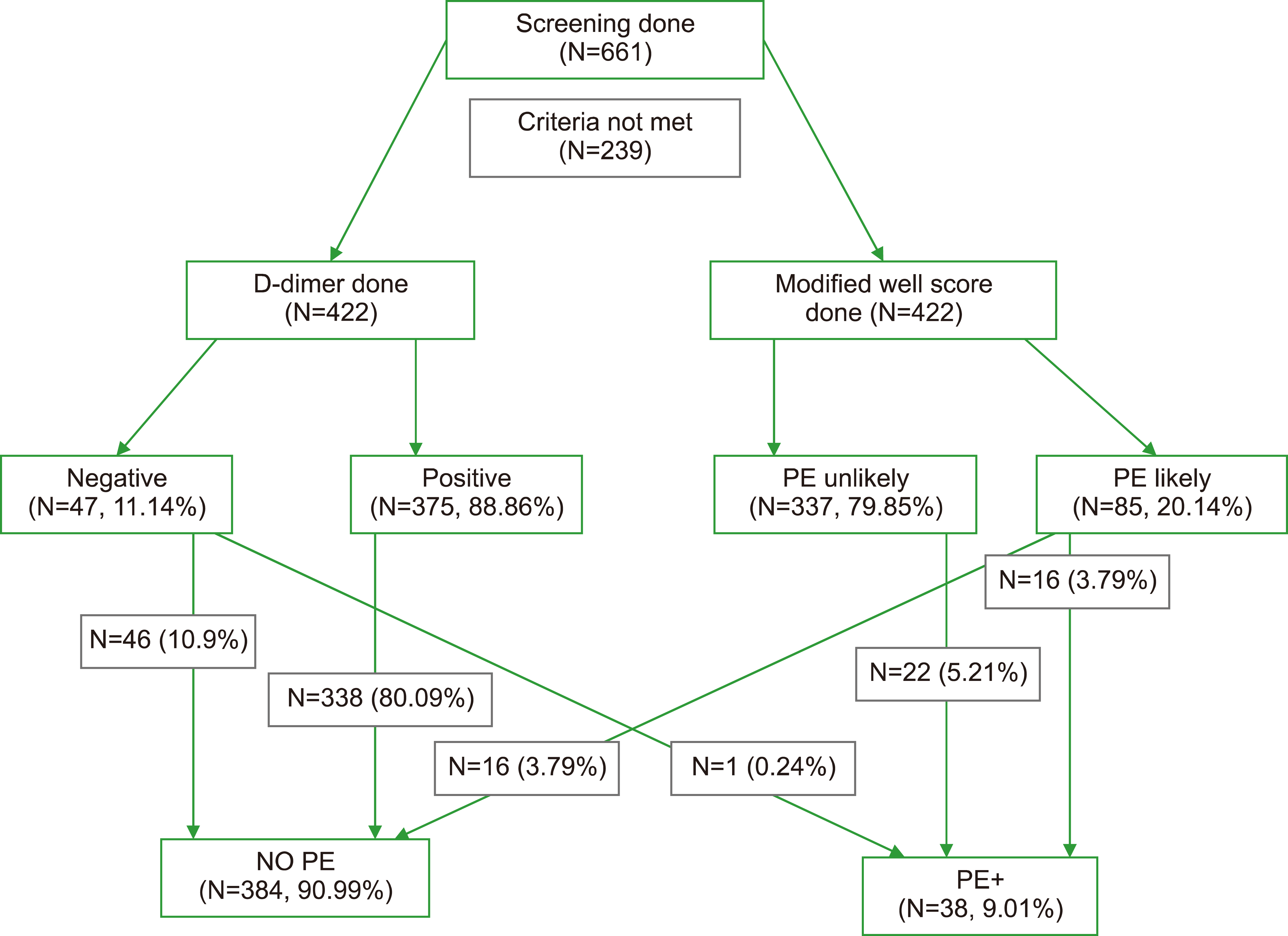
This descriptive study (cohort, retrospective electronic chart review) was conducted after receiving approval from the institutional ethics committee. From the beginning of 2010 to the end of 2018, all patients aged ≥18 years who underwent CTPA for suspected PE at the Emergency Department of King Abdulaziz Medical City in Riyadh, Saudi Arabia. Of the 661 patients screened, 422 who met the selection criteria were included in this study (Fig. 1). The patients were identified by reviewing all CTPA requests in the electronic case and radiology databases. This study excluded participants under 18 years of age, pregnant women, and those with known antiphospholipid syndrome.
The CTPA was performed throughout the study period on GE healthcare scanners (Helical scan with 100 Kv, 7 noise index, 0.4 s rotation time, and 1.375:1 pitch and speed) and Siemens scanners (Helical scan with 100 Kv, 1.3 pitch, and 0.33 s rotation time) with Xenetix 350 contrast media. The D-dimer assay was performed using INNOVANCE (Siemens Healthcare Diagnostics, Erlangen, Germany), with a normal value defined as <0.5 mg/L. There were no hospital policies or guidelines requiring modified Wells scores (mWS) and/or D-dimer assays during the study period before requesting CTPA.
Data on demographic characteristics, comorbidities, presenting symptoms, and physical examination results were obtained from the electronic health records. Additionally, we obtained the pretest probability for venous thrombosis at presentation prior to CTPA, as well as the initial laboratory report of the thrombophilia test, CTPA result, and any post-CTPA complications.
Pretest risk scores for PE were calculated retrospectively for each patient using the mWS and D-dimer level with the investigators blinded to the CTPA results. The mWS was classified as PE unlikely (mWS ≤4) or PE likely (mWS ≥4). To determine the criteria for an “alternative diagnosis less likely than PE” in the Wells score retrospectively, we considered whether the prescriber had the sole diagnosis of PE or the first differential diagnosis in the document prior to requesting CTPA. Avoidable CTPA was defined in this study as unlikely PE (mWS ≤4) with a negative D-dimer.
IBM SPSS version 25 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Demographic and clinical characteristics were analyzed using descriptive statistics, while categorical variables were analyzed using contingency table analysis and t-tests.
Of the 661 patients screened, 422 who met the selection criteria and were suspected of having PE underwent CTPA. The mean age was 59.21 (±19.48) years, D-dimer was 2.79 (±4.58) g/L, and the majority of the participants were female (N=311, 73.70%). Diabetes mellitus (36.5%) and hypertension (40.52%) were all common comorbidities. Other common comorbidities included heart failure (9.95%) and cardiovascular diseases (12.3%). Chest pain (59.9%), dyspnea (51.4%), and cough (17.3%) were the frequently reported presenting symptoms. Of 661 patients, 79 (11.9%) had PE on CTPA [64 (9.7%) had segmental PE and 15 (2.3%) had segmental lobar PE] (Supplementary Table 1). The relationships of PE in CTPA and clinical characteristics of patients meeting the selection criteria are given in Table 1.
Five hundred twenty-six (79.6%) of all screened patients had an mWS of ≤4 (PE unlikely) and 422 had a D-dimer assay done (63.8%). In routine practice, the pretest probability for PE was documented in only 15 cases (2.3%). A total of 422 (63.8% of screened) patients met the selection criteria of the study, and 337 (79.86%) of them had an mWS of ≤4 (PE unlikely). A total of 22 (5.21%) patients with PE scored PE unlikely (mWS ≤4), while 16 (3.79%) scored PE likely (mWS ≥4). Similarly, 1 (0.24%) patient with PE had D-dimer negative score, while 37 (8.77%) had D-dimer positive scores (Table 2).
The mWS performance was calculated as a clinical prediction rule. Clinical prediction rules had a high specificity (82%) and low sensitivity (42.22%). Moreover, it had a high negative predictive value (93.47%) but a low positive predictive value (18.82%) (Table 2).
In this study, among those who met the selection criteria for this study, 30 (7.11%) patients had PE unlikely with negative D-dimer, which can qualify for potentially avoidable CTPA. Avoidable CTPA had 100% sensitivity, 90.3% positive predictive value, and 1.08 value in positive likelihood ratio (Table 2). In the contingency table analysis, patients with avoidable imaging had significantly more dyspnea symptoms (Table 3).
CTPA has rapidly become the first-line modality for imaging in emergency departments with suspected PE and undermines the use of clinical predictors of risk. However, an increased CTPA did not result in improved patient outcomes. Thus, there is a need to examine this issue in the clinical setting.
In this study, a significant proportion of CTPA could have been avoided if clinical predictors, such as mWS and D-dimer assay, were adequately used before imaging. However, the findings also highlight that the performance of the clinical predictors is satisfactory, and they are not used judiciously in the emergency department (for various reasons).
Demographic and clinical characteristics were comparable to those observed in previous studies [30, 31]. Although comorbid diabetes was a significant risk factor in this study, other reports from Saudi Arabia reported obesity [16] and age as risk factors [32]. This may be due to differences in the sample size and clinical characteristics of the population studied.
In the study population, 9% had PE. This is in contrast to the higher rates reported in Saudi Arabia [16, 28, 32]. However, this is consistent with other reports [31, 33], although a review and meta-analysis revealed a wide variation in prevalence [34, 35]. The estimate appears to vary according to the hospital setting [36, 37].
In contrast to another study, the results showed a low rate of prediction of PE by mWS PE likely (mWS ≥4), probably due to the retrospective study design [38]. The performance characteristics of the clinical prediction of the mWS were also low, which is consistent with a previous report [39]. The D-dimer positive value also had a lower prediction of PE, similar to an earlier account, particularly regarding non-lobar involvement [40]. In contrast to previous reports, we did not observe central artery involvement in CTPA [41-43]. Although the frequency of segmental PE observed in this study was consistent with previous reports [44-46], it frequently presented with complaints of chest pain and dyspnea [47, 48].
The results reveal avoidable (overuse) of CTPA in 7.11% when defined criteria of DD negative+PE unlikely in mWS were considered in the estimation. It had a high sensitivity (100%) and positive predictive value (90.03%), but very low specificity (7.8%) and negative predictive value (0%). This finding is partially supported by a recent study conducted during a pandemic [49]. Thus, if pretest probability estimation had been performed, a large proportion of patients could have been spared CTPA. There is no reason to believe that the pretest probability estimated in this study would have been significantly different if the same estimation was performed prior to the CTPA in a real-world scenario; thus, it is improbable that we are overestimating the frequency of avoidable CTPA. However, our estimation of avoidable CTPA was lower than those in other reports. For example, Venkatesh et al. [15] reported 38% imaging in low-risk patients in a large study, whereas Perelas et al. [10] observed a minimum of 49% avoidable imaging. One recent study with a design similar to ours observed that one-third of patients underwent CTPA clinically inappropriately [16]. There are several possible explanations for the excessive use of CTPA. Among them are concerns regarding missing potentially life-threatening PE; underestimation of adverse consequences associated with the use of CT, the ease with which this mode of investigation can be used; and an insufficient policy for utilizing prediction rules [17, 18, 50].
In this study, dyspnea symptoms appeared in significantly more patients in the avoidable CTPA group. This finding is partially supported by other reports that dyspnea symptoms are associated with a high rate of CTPA use despite a low positive rate, and a substantial proportion may have other explanations for dyspnea [51-54].
One should be clear that CTPA in suspected PE as it provides rapid and accurate diagnosis, allowing for timely initiation of appropriate treatment, hence preventing significant morbidity and mortality and complications, such as right heart failure, pulmonary hypertension, or even death. Physician anxiety regarding PE, barriers to using the evidence, divergent views on evidence-based PE testing, inherent Wells score problems, the drive to obtain CT rather than diagnose PE, subjective reasoning and cognitive biases supporting deviation from evidence-based tests, and the use of evidence-based testing to rule out PE in very unlikely patients all influenced CT test choices for PE [55].
This study should be interpreted in light of the fact that it was retrospective, and the pretest risk score for PE was estimated accordingly, which may not be exact in all cases due to the nature of clinical symptoms and signs. It likely did not capture the legitimate reason for prescribing CTPA by the concerned clinician, and other associated characteristics may have conferred a high risk of PE.
Underutilization of clinical prediction rules is prevalent, and its use may decrease CTPA overuse. A mandatory policy requiring emergency departments to evaluate avoidable CTPA imaging may reduce CTPA overuse and the associated burden on patients and the healthcare system.
REFERENCES
1. Morris T, Fedullo P. 2016; Pulmonary thromboembolism. In: Courtney Broaddus V, Mason RJ, Ernst JD, et al, eds. Murray and Nadel's textbook of respiratory medicine. 6th ed. Philadelphia, PA:. Elsevier Saunders,. 1001. DOI: 10.1016/B978-1-4557-3383-5.00057-9. PMID: 27324830.
2. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. 1998; Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 158:585–93. DOI: 10.1001/archinte.158.6.585. PMID: 9521222.

3. AlEidan FAS, AlManea RK, AlMoneef AT, et al. 2022; Incidence and predictors of recurrence and mortality following first venous thromboembolism among the Saudi population: single-center cohort study. Int J Gen Med. 15:7559–68. DOI: 10.2147/IJGM.S359893. PMID: 36199587. PMCID: PMC9527814.
4. El Margoushy N, Al-Suwat R, Al-Otaibi W, Mougrabi M. 2017; Incidence of pulmonary embolism in CCU at King Faisal Hospital, Taif, Saudi Arabia. Egypt J Hosp Med. 68:865–77. DOI: 10.12816/0038185.
5. Alharbi SH. 2020; Incidence of pulmonary thromboembolism and its associated comorbidities in Ha'il Region, Northern Saudi Arabia. Med Sci. 24:4352–8.
6. Algahtani FH, Bayoumi N, Abdelgadir A, et al. 2013; Clinical characteristics and risk factors of pulmonary embolism: data from a Saudi tertiary-care center. J Thromb Haemost. 11:388–90. DOI: 10.1111/jth.12083. PMID: 23205904.
7. Aziz S, Mfarah S, Rehman A, et al. 2020; Epidemiology and pattern of thromboembolism in Aseer Central Hospital, a multispecialty hospital in Aseer region, Abha in Southern Saudi Arabia. J Cardiol Cardiovas Res. 1:1–12. DOI: 10.37191/mapsci-jccr-1(2)-017.
8. Stein PD, Fowler SE, Goodman LR, et al. 2006; Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 354:2317. DOI: 10.1056/NEJMoa052367. PMID: 16738268.
9. Mamlouk MD, vanSonnenberg E, Gosalia R, et al. 2010; Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology. 256:625. DOI: 10.1148/radiol.10091624. PMID: 20551182.
10. Perelas A, Dimou A, Saenz A, et al. 2015; CT pulmonary angiography utilization in the emergency department: diagnostic yield and adherence to current guidelines. Am J Med Qual. 30:571–7. DOI: 10.1177/1062860614543302. PMID: 25037560.
11. Crichlow A, Cuker A, Mills AM. 2012; Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med. 19:1219–26. DOI: 10.1111/acem.12012. PMID: 23167851. PMCID: PMC3506180.
12. Molaee S, Ghanaati H, Safavi E, Foroumandi M, Peiman S. 2015; Computed tomography pulmonary angiography for evaluation of patients with suspected pulmonary embolism: use or overuse. Iran J Radiol. 12:e22383. DOI: 10.5812/iranjradiol.12(2)2015.22383. PMID: 26557282.

13. Burge AJ, Freeman KD, Klapper PJ, Haramati LB. 2008; Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 63:381–6. DOI: 10.1016/j.crad.2007.10.004. PMID: 18325357.
14. Haap MM, Gatidis S, Horger M, Riessen R, Lehnert H, Haas CS. 2012; Computed tomography angiography in patients with suspected pulmonary embolism-too often considered? Am J Emerg Med. 30:325–30. DOI: 10.1016/j.ajem.2010.12.013. PMID: 21277141.

15. Venkatesh AK, Kline JA, Courtney DM, et al. 2012; Evaluation of pulmonary embolism in the emergency department and consistency with a national quality measure: quantifying the room for improvement. Arch Intern Med. 172:1028–32. DOI: 10.1001/archinternmed.2012.1804. PMID: 22664742. PMCID: PMC3775003.
16. Al Dandan O, Hassan A, Alnasr A, et al. 2020; The use of clinical decision rules for pulmonary embolism in the emergency department: a retrospective study. Int J Emerg Med. 13:23. DOI: 10.1186/s12245-020-00281-1. PMID: 32393324. PMCID: PMC7216540. PMID: 4562014cd64845f5b013692d278d2e63.
17. Takahashi EA, Yoon HC. 2013; Four-year cumulative radiation exposure in patients undergoing computed tomography angiography for suspected pulmonary embolism. Radiol Res Pract. 2013:482403. DOI: 10.1155/2013/482403. PMID: 23984065. PMCID: PMC3745975. PMID: b7b3369870194449abe46d3062496ac0.
18. Mitchell AM, Jones AE, Tumlin JA, Kline JA. 2012; Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med. 19:618–25. DOI: 10.1111/j.1553-2712.2012.01374.x. PMID: 22687176. PMCID: PMC5366244.

19. Lecumberri R, Alfonso A, Jiménez D, et al. 2013; Dynamics of case-fatalilty rates of recurrent thromboembolism and major bleeding in patients treated for venous thromboembolism. Thromb Haemost. 110:834–43. DOI: 10.1160/TH13-02-0132. PMID: 23846721.
20. Wells PS, Anderson DR, Rodger M, et al. 2000; Derivation of a simple clinical model to categorize patients' probability of pulmonary embolism: increasing the model's utility with the SimpliRED D-dimer. Thromb Haemost. 83:416–20. DOI: 10.1055/s-0037-1613830. PMID: 10744147.

21. Wells PS, Anderson DR, Rodger M, et al. 2001; Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 135:98–107. DOI: 10.7326/0003-4819-135-2-200107170-00010. PMID: 11453709.
22. Kline JA, Courtney DM, Kabrhel C, et al. 2008; Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 6:772–80. DOI: 10.1111/j.1538-7836.2008.02944.x. PMID: 18318689.
23. Konstantinides SV, Meyer G, Becattini C, et al. 2020; 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 41:543–603. DOI: 10.1093/eurheartj/ehz405. PMID: 31504429.
24. Righini M, Perrier A, De Moerloose P, Bounameaux H. 2008; D-dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 6:1059–71. DOI: 10.1111/j.1538-7836.2008.02981.x. PMID: 18419743.
25. Pasha SM, Klok FA, Snoep JD, et al. 2010; Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 125:e123–7. DOI: 10.1016/j.thromres.2009.11.009. PMID: 19942258.
26. United States Government Accountability Office. 2008. Medicare part B imaging services: rapid spending growth and shift to physician offices indicate need for CMS to consider additional management practices. Government Accountability Office;Washington, D.C.: https://www.gao.gov/products/gao-08-452. Accessed December 20, 2022.
27. Mitchell AM, Jones AE, Tumlin JA, Kline JA. 2011; Immediate complications of intravenous contrast for computed tomography imaging in the outpatient setting are rare. Acad Emerg Med. 18:1005–9. DOI: 10.1111/j.1553-2712.2011.01152.x. PMID: 21854485.
28. Owaidah T, AlGhasham N, AlGhamdi S, et al. 2014; Evaluation of the usefulness of a D dimer test in combination with clinical pretest probability score in the prediction and exclusion of venous thromboembolism by medical residents. Thromb J. 12:28. DOI: 10.1186/s12959-014-0028-7. PMID: 25530719. PMCID: PMC4272774.

29. Abolfotouh MA, Almadani K, Al Rowaily MA. 2020; Diagnostic accuracy of D-dimer testing and the revised Geneva score in the prediction of pulmonary embolism. Int J Gen Med. 13:1537–43. DOI: 10.2147/IJGM.S289289. PMID: 33363402. PMCID: PMC7751841.
30. Anwar A, Wong HS, Muza R. 2017; Audit on the use of CTPA in pulmonary embolism (PE) diagnosis. Gen Int Med Clin Innov. 2:1–5. DOI: 10.15761/GIMCI.1000144.
31. Perera M, Aggarwal L, Scott IA, Cocks N. 2017; Underuse of risk assessment and overuse of computed tomography pulmonary angiography in patients with suspected pulmonary thrombo-embolism. Intern Med J. 47:1154–60. DOI: 10.1111/imj.13524. PMID: 28635149.

32. Alharbi SH, Almegren M, Alshammari HA, et al. 2020; Appropriateness of pulmonary CT angiography testing request in patients suspected with pulmonary embolism in Hai'l region, Northern Saudi Arabia. Med Sci. 24:4190–5.
33. Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. 2020; Over-testing for suspected pulmonary embolism in American emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 13:e005753. DOI: 10.1161/CIRCOUTCOMES.119.005753. PMID: 31957477.
34. Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. 2013; Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism--revisited: a systematic review and meta-analysis. Emerg Med J. 30:701–6. DOI: 10.1136/emermed-2012-201730. PMID: 23038695.
35. Germini F, Zarabi S, Eventov M, Turcotte M, Li M, de Wit K. 2019; Prevalence of pulmonary embolism in patients accessing the emergency department, reported by country of study: a systematic review and meta-analysis. Res Pract Thromb Haemost (ISTH 2020 Congress Abstracts). 4(Suppl 1):PB2343. DOI: 10.1016/j.thromres.2019.09.013.
36. Wiener RS, Schwartz LM, Woloshin S. 2011; Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 171:831–7. DOI: 10.1001/archinternmed.2011.178. PMID: 21555660. PMCID: PMC3140219.

37. Hutchinson BD, Navin P, Marom EM, Truong MT, Bruzzi JF. 2015; Overdiagnosis of pulmonary embolism by pulmonary CT angiography. AJR Am J Roentgenol. 205:271–7. DOI: 10.2214/AJR.14.13938. PMID: 26204274.
38. Alhassan S, Sayf AA, Arsene C, Krayem H. 2016; Suboptimal implementation of diagnostic algorithms and overuse of computed tomography-pulmonary angiography in patients with suspected pulmonary embolism. Ann Thorac Med. 11:254–60. DOI: 10.4103/1817-1737.191875. PMID: 27803751. PMCID: PMC5070434. PMID: 9b7d8ad63f7442789d1b3ce064b370be.
39. Obradović D, Joveš B, Pena Karan S, Stefanović S, Ivanov I, Vukoja M. 2016; Correlation between the Wells score and the Quanadli index in patients with pulmonary embolism. Clin Respir J. 10:784–90. DOI: 10.1111/crj.12291. PMID: 25763885.
40. Kubak MP, Lauritzen PM, Borthne A, Ruud EA, Ashraf H. 2016; Elevated d-dimer cut-off values for computed tomography pulmonary angiography-d-dimer correlates with location of embolism. Ann Transl Med. 4:212. DOI: 10.21037/atm.2016.05.55. PMID: 27386486. PMCID: PMC4916352.
41. Alonso Martinez JL, Anniccherico Sánchez FJ, Urbieta Echezarreta MA, García IV, Álvaro JR. 2016; Central versus peripheral pulmonary embolism: analysis of the impact on the physiological parameters and long-term survival. N Am J Med Sci. 8:134–42. DOI: 10.4103/1947-2714.179128. PMID: 27114970. PMCID: PMC4821092.
42. Laporte S, Mismetti P, Décousus H, et al. 2008; Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 117:1711–6. DOI: 10.1161/CIRCULATIONAHA.107.726232. PMID: 18347212.
43. Torres-Macho J, Mancebo-Plaza AB, Crespo-Giménez A, et al. 2013; Clinical features of patients inappropriately undiagnosed of pulmonary embolism. Am J Emerg Med. 31:1646–50. DOI: 10.1016/j.ajem.2013.08.037. PMID: 24060320.
44. Carrier M, Righini M, Le Gal G. 2012; Symptomatic subsegmental pulmonary embolism: what is the next step? J Thromb Haemost. 10:1486–90. DOI: 10.1111/j.1538-7836.2012.04804.x. PMID: 22672341.

45. Carrier M, Righini M, Wells PS, et al. 2010; Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 8:1716–22. DOI: 10.1111/j.1538-7836.2010.03938.x. PMID: 20546118.

46. Goy J, Lee J, Levine O, Chaudhry S, Crowther M. 2015; Sub-segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 13:214–8. DOI: 10.1111/jth.12803. PMID: 25442511.

47. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014; 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 35:3033–69. 3069a–k. DOI: 10.1093/eurheartj/ehu283. PMID: 25173341.
48. Miniati M, Cenci C, Monti S, Poli D. 2012; Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One. 7:e30891. DOI: 10.1371/journal.pone.0030891. PMID: 22383978. PMCID: PMC3288010. PMID: c10de22305584a958a25052c69a780bf.

49. Raj K, Chandna S, Doukas SG, et al. 2021; Combined use of wells scores and D-dimer levels for the diagnosis of deep vein thrombosis and pulmonary embolism in COVID-19: a retrospective cohort study. Cureus. 13:e17687. DOI: 10.7759/cureus.17687. PMID: 34650862. PMCID: PMC8487632.

50. Rohacek M, Buatsi J, Szucs-Farkas Z, et al. 2012; Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med. 38:1345–51. DOI: 10.1007/s00134-012-2595-z. PMID: 22584801.
51. Donohoo JH, Mayo-Smith WW, Pezzullo JA, Egglin TK. 2008; Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr. 32:421–5. DOI: 10.1097/RCT.0b013e31812e6af3. PMID: 18520550.

52. Shujaat A, Shapiro JM, Eden E. 2013; Utilization of CT pulmonary angiography in suspected pulmonary embolism in a major urban emergency department. Pulm Med. 2013:915213. DOI: 10.1155/2013/915213. PMID: 24078873. PMCID: PMC3783975. PMID: 73c99a678556460db491011c4b106c12.

53. Green DB, Raptis CA, Alvaro Huete Garin I, Bhalla S. 2015; Negative computed tomography for acute pulmonary embolism: important differential diagnosis considerations for acute dyspnea. Radiol Clin North Am. 53:789–99. ixDOI: 10.1016/j.rcl.2015.02.014. PMID: 26046511.
54. Ozakin E, Kaya FB, Acar N, Cevik AA. 2014; An analysis of patients that underwent computed tomography pulmonary angiography with the prediagnosis of pulmonary embolism in the emergency department. ScientificWorldJournal. 2014:470358. DOI: 10.1155/2014/470358. PMID: 24955406. PMCID: PMC4052481. PMID: e2da53490157409fb400168e37e7089d.

55. Zarabi S, Chan TM, Mercuri M, et al. 2021; Physician choices in pulmonary embolism testing. CMAJ. 193:E38–46. DOI: 10.1503/cmaj.201639. PMID: 33431544. PMCID: PMC7773048.

Table 1
Relationships of PE in CTPA and clinical variables in patients meeting selection criteria.
Table 2
Performance characteristics of modified Wells score and estimated avoidable CTPA with actual CTPA results.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download