Abstract
Background
The PLASMIC score is a convenient tool for predicting ADAMTS13 activity of <10%. Lactate dehydrogenase (LDH) is widely used as a marker of haemolysis in thrombotic thrombocytopenic purpura (TTP) monitoring, and could be used as a replacement marker for lysis. We aimed to validate the PLASMIC score in a multi-centre Asia Pacific region, and to explore whether LDH could be used as a replacement marker for lysis.
Methods
Records of patients with thrombotic microangiopathy (TMA) were reviewed. Patients’ ADAMTS13 activity levels were obtained, along with clinical/laboratory findings relevant to the PLASMIC score. Both PLASMIC scores and PLASMIC-LDH scores, in which LDH replaced traditional lysis markers, were calculated. We generated a receiver operator characteristics (ROC) curve and compared the area under the curve values (AUC) to determine the predictive ability of each score.
Results
46 patients fulfilled the inclusion criteria, of which 34 had ADAMTS13 activity levels of <10%. When the patients were divided into intermediate-to-high risk (scores 5‒7) and low risk (scores 0‒4), the PLASMIC score showed a sensitivity of 97.1% and specificity of 58.3%, with a positive predictive value (PPV) of 86.8% and negative predictive value (NPV) of 87.5%. The PLASMIC-LDH score had a sensitivity of 97.1% and specificity of 33.3%, with a PPV of 80.5% and NPV of 80.0%.
Thrombotic thrombocytopenic purpura (TTP) is a life- threatening haematologic emergency characterized by a deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) protease. This deficiency leads to the accumulation of ultra-large von Willebrand factor (VWF), microvascular thrombosis, and microangiopathic haemolytic anaemia (MAHA) [1-4]. Clinical suspicion of TTP should be made due to the presence of schistocytes, anaemia, and thrombocytopenia (MAHA) visualized by blood film examination. A severe deficiency of ADAMTS13, at an activity level of <10%, can constitute a diagnosis of TTP [3-6]. However, it is not a routine investigation performed in many hospital laboratories, and results from a reference laboratory may delay treatment [7].
The PLASMIC score is a predictive tool to risk-stratify the likelihood of a severe deficiency of ADAMTS-13 activity, of <10% [8, 9]. The score utilizes seven key clinical and routine laboratory components, with one point allocated for platelet count <30×109/L, presence of lysis markers (reticulocyte count >2.5%, indirect bilirubin >2.0 mL/dL or undetectable haptoglobin), absence of active malignancy, no solid organ or stem cell transplantation, mean cell volume (MCV) of <90 fL, international normalized ratio (INR) <1.5, and creatinine <2 mg/dL, which it then uses to assign patients as having either low (0–4), intermediate (5) or high risk (6–7).
Lactate dehydrogenase (LDH) is a routine biomarker used in the monitoring of the clinical response to TTP treatment. Unlike the traditional markers used in the PLASMIC score, LDH is a non-specific marker of cell lysis. Previous studies have reported higher LDH levels in patients with TTP compared to non-TTP patients with thrombotic microangiopathy (TMA), and have utilized LDH in different ways to predict the likelihood of severe TTP, with encouraging correlation results [10, 11]. We therefore explored the possibility of simplifying the PLASMIC score by using a higher LDH level (twice the normal upper limit) as a potential replacement for the traditional lysis markers (reticulocyte count, haptoglobin, and indirect bilirubin).
Patient records were retrospectively obtained from 16 centre across seven countries in the Asia Pacific region (Australia, Malaysia, New Zealand, Thailand, Singapore, Korea, and Hong Kong) for cases between 2001 to 2020. All identified patients were adults and had clinical suspicions of thrombotic microangiopathy (TMA). ADAMTS13 activity assays at the respective sites were assessed using chromogenic enzyme-linked immunosorbent assay (ELISA) or fluorescence resonance energy transfer (FRET). Thrombotic thrombocytopenic purpura (TTP) was defined as a severe deficiency of ADAMTS13 with an activity level of <10%, and the presence or absence of an inhibitor was also recorded.
We excluded patients without ADAMTS13 results and missing clinical or laboratory components necessary to make the PLASMIC evaluation. Patients were not excluded if they had at least one marker for haemolysis available (reticulocyte count, haptoglobin, and indirect bilirubin). Patients were also excluded if they had an ADAMTS13 activity level ≥10%, and if sampling was performed after they had received treatment via plasma products infusion or plasma exchange.
We performed a logistic regression analysis and Fisher’s exact test on the PLASMIC variables, together with LDH, to compare these factors between patients with TTP (ADAMTS13 activity <10%) and controls with higher levels of activity (ADAMTS13 activity ≥10%).
As part of this study, we also aimed to validate the PLASMIC score in our cohort. All patients had their PLASMIC scores calculated, and were each assigned to one of two risk categories: the low risk (0–4) group, and the intermediate- (5) to-high (6–7) group. We also generated an alternative scoring model, dubbed “PLASMIC-LDH” using the same parameters used in the original PLASMIC scores, but substituting an LDH level of >2x the normal upper limit (NUL) to assess the presence of haemolysis, and assigned the patients to the risk groups in a similar fashion. This threshold was chosen based on observations of patients with severe ADAMTS13 deficiencies in previous reports [10, 11].
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for both models were calculated. Descriptive analysis was performed using the statistical analysis package of R v3.6.2 (https://www.r-project.org). All continuous variables detailed in this report are expressed as median (interquartile range), and categorical variables are expressed as number/total (%). We generated a receiver operator characteristics (ROC) curve and compared the area under the curve values (AUC) using DeLong’s test to determine the predictive ability of each model.
Records from a total of 72 patients with suspected TMA were collected from the various centres. A total of 26 patients were excluded: 14 who were missing ADAMTS13 results, one with a post-plasma exchange ADAMTS13 result, and 11 who were missing PLASMIC score criteria. The remaining 46 patients were included and formed the study cohort (Fig. 1).
The median age at presentation was 50 years [interquartile range (IQR), 35–54]. The cohort comprised 27 females and 19 males. In terms of racial demographics, there were 27 patients of Asian ethnicity (23 Southeast Asian and four East Asian), 17 Caucasians, and two patients of other ethnicities. The prevalence of TTP was 73.9% (N=34), and of these patients 61.8% (N=21) had a documented presence of an inhibitor. Central nervous system (CNS) manifestations were the most common clinical symptom 71.7% (N=33). Demographic features and clinical and laboratory data are summarised in Table 1.
We analysed all the components of the PLASMIC score, as well as LDH, and compared them between patients with and without TTP. Both the haemolysis markers and an elevated LDH level were found to be associated with more than a 2-fold increased risk of having TTP, and were more frequently observed in the TTP group (61.8% vs. 41.7% for lysis markers, and 85.3% vs. 66.7% for elevated LDH). However, both variables were not statistically significant. Only thrombocytopenia (<30×109/L) and preserved renal function (<2 mg/dL) were identified as variables that were significantly different between the two groups (Table 2, 3).
Validation of the PLASMIC score was done first. Of the 46 patients, 28 were assigned to the high-risk (6–7 points) group, 10 to the intermediate (5 points) group, and eight to the low-risk (0–4 points) group. We then substituted LDH into the PLASMIC score. The modified PLASMIC-LDH score was determined for all 46 patients. In this model, 32 were assigned to the high-risk (6–7 points) group, nine to the intermediate (5 points) group, and five to the low-risk (0–4 points) group (Table 4).
When divided into intermediate-high risk and low risk, the PLASMIC score had a sensitivity of 97.1% and specificity of 58.3%, with a PPV of 86.8%, and an NPV of 87.5%. Using the same split of intermediate-high risk (scores 5–7) and low risk (scores 0–4), the PLASMIC-LDH had a sensitivity of 97.1%, a specificity of 33.3%, a PPV of 80.5%, and an NPV of 80.0% (Table 5).
A receiver operator characteristics (ROC) curve constructed for both the PLASMIC and PLASMIC-LDH scores showed that the difference in the area under the curve value (AUC) for PLASMIC (0.841) and PLASMIC-LDH (0.852) was not statistically significant (P=0.724) (Fig. 2).
The use of a pre-test probability tool such as PLASMIC or the French Mortality in TTP Score (MITS) (Benhamou et al., 2012 [12]) to identify those with a high risk of severe TTP has been reinforced in the latest treatment guidelines [6]. Studies have reported that a high PLASMIC score predicts a good response to plasma exchange (PEX) and a better long-term survival outcome, as it is highly suggestive of TTP, while a low score indicates an alternate diagnosis with a poorer response to PEX and higher probability of mortality [9, 12]. This is supported by findings of studies in which patients with severe ADAMTS13 deficiency (<10%) had better outcomes compared to those with higher levels [13, 14].
Our study was performed in part to validate the PLASMIC score in the Asia Pacific region. Our study results were compared favourably to the PLASMIC derivation cohort by Bendapudi et al. [8, 9] (sensitivity of 98.7% and specificity of 63.0%, with a PPV of 53.6% and an NPV of 99.1%). A recent meta-analysis involving 970 patients from 13 studies concurred with the diagnostic accuracy of the PLASMIC score, reporting the intermediate- and high-risk score (5–7) as having a sensitivity of 99% and specificity of 57%, with an NPV of 99%, in a study pool where the prevalence of TTP was 35% [15].
Despite these findings, the PLASMIC score is not widely applied in clinical practice, perhaps due to the lack of immediate availability of investigation results at patient presentation and poor specificity in certain population cohorts [10]. Liu et al., [16] for example, reported a reduced sensitivity of the PLASMIC score in older populations.
In the original derivation and validation cohort of the PLASMIC score, Bendapudi et al. [8, 9] reported that the degree LDH elevation did not add value to the score. However, several other studies have explored alternative methods, particularly with regard to the the use of LDH to assist in predicting high-risk groups with TTP [10, 11]. We hypothesized that using LDH, which is more readily available, would make the PLASMIC score a more convenient tool to use.
Our study findings, of a reduced specificity in our PLASMIC-LDH model, did not significantly impair its overall predictive ability. The reduced specificity is most likely attributed to LDH being a non-specific marker of cell lysis, compared to the traditional haemolytic markers used in the PLASMIC score. However, given the high sensitivity rate (97.1%), it is still a useful tool for identifying high-risk patients for treatment in the absence of conventional haemolysis markers.
It is also worth noting that the PLASMIC score was derived and widely validated to predict an ADAMTS13 level of <10%. In our study, we excluded two patients from our TTP group who had borderline-intermediate ADAMTS13 levels (10–20%), and who did achieve complete responses with plasma exchange. In their study, Li et al. [13] demonstrated that it is feasible to extrapolate the use of the PLASMIC score to a higher ADAMTS13 level of 15%, in order to include more patients in the borderline group who may benefit from treatment. By using a similar threshold of 15% in our cohort, the PLASMIC score had a sensitivity of 97.2% and specificity of 70%, with a PPV of 92.1% and an NPV of 87.5%.
Our study had a few limitations. Firstly, the final sample size obtained (N=46) through our stringent selection criteria was small, even for an infrequently encountered haematological disease. We could have potentially overestimated the prevalence of severe TTP (73.9%) through the use of our exclusion criteria. Secondly, we lacked the complete markers for haemolysis required for the evaluation of lysis in the PLASMIC score. Only three patients had complete sets of all three lysis markers (reticulocyte count, haptoglobin, and indirect bilirubin) investigated, while the remaining 43 patients were evaluated based on combinations of two of these markers. While this may have led to underestimating the risk group, we believe that the effect was minimal given the performance of the PLASMIC score in our cohort. Moreover, we believe that this limitation actually reflects a real-world situation in which a complete set of investigations are not always available.
In conclusion, our study validated the PLASMIC score as an excellent pre-test probability tool for TTP in the Asia Pacific cohort. If all parameters are available, it should be preferentially used in patients with TMA, to guide treatment decisions. Substituting LDH into the score (PLASMIC-LDH), did not significantly impair the predictive ability of the PLASMIC score, despite a reduced specificity. It may, therefore, be reasonable to use LDH as an alternative in the absence of lysis markers, to help identify high-risk patients and empirically treat them via plasma exchange. Further validation studies of this notion are recommended.
ACKNOWLEDGMENTS
The authors would like to thank the investigators of the Asia Pacific Microangiopathic Thrombocytopenia (APMAT) network for contributing to this study.
REFERENCES
1. Furlan M, Robles R, Galbusera M, et al. 1998; von Willebrand factor- leaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 339:1578–84. DOI: 10.1056/NEJM199811263392202. PMID: 9828245.

2. Tsai HM, Lian EC. 1998; Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 339:1585–94. DOI: 10.1056/NEJM199811263392203. PMID: 9828246. PMCID: PMC3159001.

3. Sarode R, Bandarenko N, Brecher ME, et al. 2014; Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J Clin Apher. 29:148–67. DOI: 10.1002/jca.21302. PMID: 24136342.

4. Tsai HM. 2003; Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am Soc Nephrol. 14:1072–81. DOI: 10.1097/01.ASN.0000060805.04118.4C. PMID: 12660343.

5. Scully M, Hunt BJ, Benjamin S, et al. 2012; Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 158:23–35. DOI: 10.1111/j.1365-2141.2012.09167.x. PMID: 22624596.

6. Zheng XL, Vesely SK, Cataland SR, et al. 2020; ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 18:2486–95. DOI: 10.1111/jth.15006. PMID: 32914582. PMCID: PMC8146131.

7. Martin IW, Katus MC, Martin CL, Szczepiorkowski ZM, Gorham JD, Dunbar NM. 2016; Rapid ADAMTS13 availability impacts treatment for microangiopathic hemolytic anemia and thrombocytopenia. J Clin Apher. 31:419–22. DOI: 10.1002/jca.21419. PMID: 26332753.

8. Bendapudi PK, Li A, Hamdan A, et al. 2014; Derivation and prospective validation of a predictive score for the rapid diagnosis of thrombotic thrombocytopenic purpura: the plasmic score. Blood (ASH Annual Meeting Abstracts). 124(Suppl):231. DOI: 10.1182/blood.V124.21.231.231.

9. Bendapudi PK, Hurwitz S, Fry A, et al. 2017; Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 4:e157–64. DOI: 10.1016/S2352-3026(17)30026-1. PMID: 28259520.

10. Zhao N, Zhou L, Hu X, et al. 2020; A modified PLASMIC score including the lactate dehydrogenase/the upper limit of normal ratio more accurately identifies Chinese thrombotic thrombocytopenic purpura patients than the original PLASMIC score. J Clin Apher. 35:79–85. DOI: 10.1002/jca.21760. PMID: 31724781.

11. Tang N, Wang X, Li D, Sun Z. 2018; Validation of the PLASMIC score, a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis, in Chinese patients. Thromb Res. 172:9–13. DOI: 10.1016/j.thromres.2018.10.010. PMID: 30340093.

12. Benhamou Y, Assié C, Boelle PY, et al. 2012; Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombo-cytopenic purpura: the French TMA Reference Center experience. Haematologica. 97:1181–6. DOI: 10.3324/haematol.2011.049676. PMID: 22580997. PMCID: PMC3409815.

13. Li A, Khalighi PR, Wu Q, Garcia DA. 2018; External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. 16:164–9. DOI: 10.1111/jth.13882. PMID: 29064619. PMCID: PMC5760324.

14. Yap YY, Sathar J, Law KB, et al. 2018; Clinical characteristics and outcomes of thrombotic microangiopathy in Malaysia. Blood Res. 53:130–7. DOI: 10.5045/br.2018.53.2.130. PMID: 29963519. PMCID: PMC6021566.

15. Paydary K, Banwell E, Tong J, Chen Y, Cuker A. 2020; Diagnostic accuracy of the PLASMIC score in patients with suspected thrombotic thrombocytopenic purpura: a systematic review and meta-analysis. Transfusion. 60:2047–57. DOI: 10.1111/trf.15954. PMID: 32757237.
16. Liu A, Dhaliwal N, Upreti H, et al. 2021; Reduced sensitivity of PLASMIC and French scores for the diagnosis of thrombotic thrombocytopenic purpura in older individuals. Transfusion. 61:266–73. DOI: 10.1111/trf.16188. PMID: 33179792. PMCID: PMC8859842.

Fig. 1
Study cohort design outlining the method of patient selection for analysis. The total PLASMIC/ PLASMIC LDH score was calculated with one point designated for each component and subsequently stratified into low risk (0–4 points), intermediate risk (5 points), and high risk (6–7 points).
Fig. 2
DeLong’s test for the receiver operator curve (ROC) with a comparison of the area under the curve (AUC) of PLASMIC (black) and PLASMIC-LDH (blue).
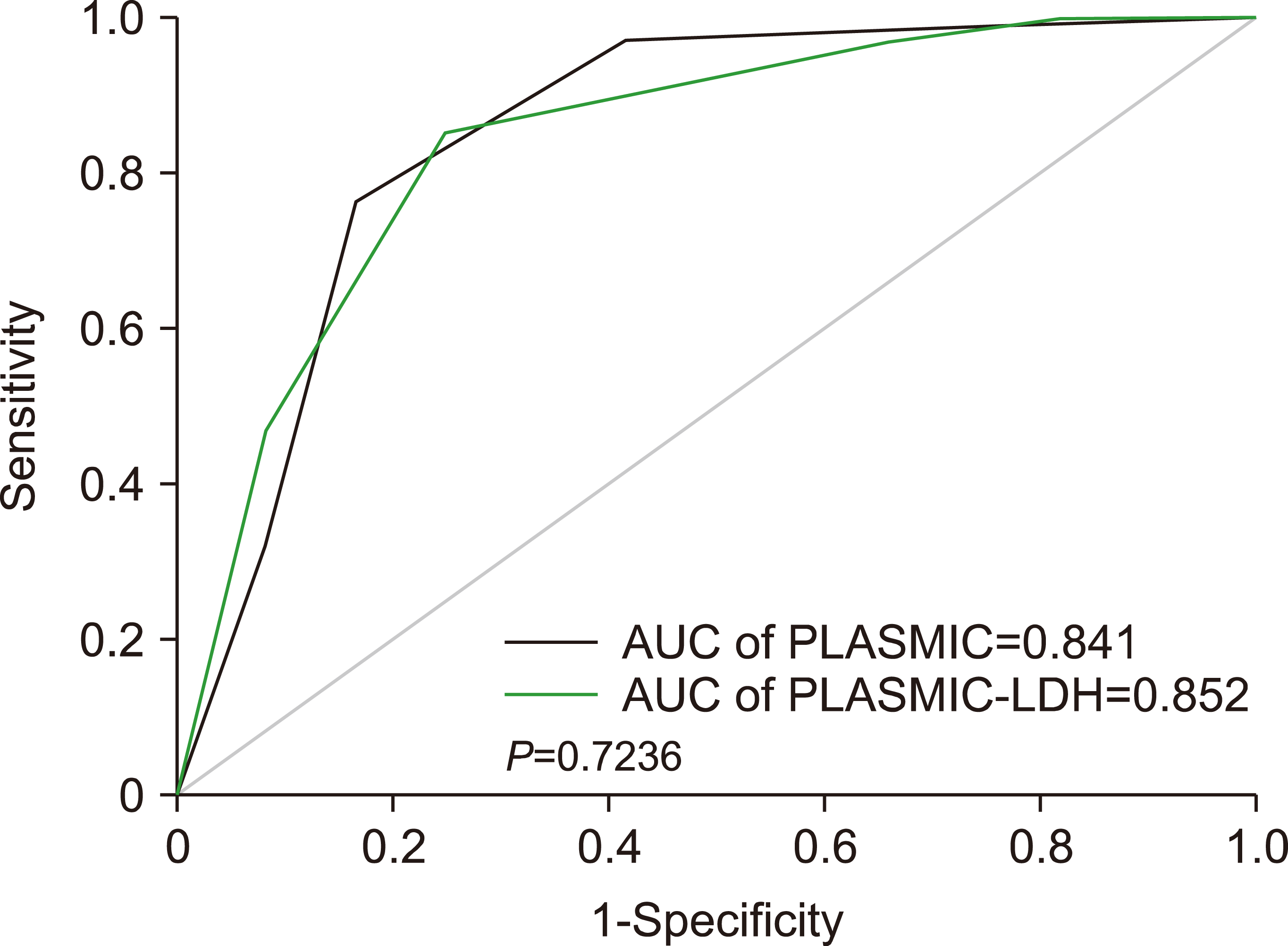
Table 1
Patient baseline clinical characteristics.
Table 2
Comparison of the PLASMIC variables between TTP (ADAMTS13 <10%) and non-TTP (ADAMTS13 ≥10%).
Table 3
Logistic regression analysis of factors associated with TTP (ADAMTS13 <10%).
Table 4
Patient risk stratification based on both PLASMIC and PLASMIC-LDH models.
| Risk Stratification | PLASMIC (N=46) | PLASMIC LDH (N=46) |
|---|---|---|
| High (6–7 points) | 28 (60.9%) | 32 (69.6%) |
| Intermediate (5 points) | 10 (21.7%) | 9 (19.6%) |
| Low (0–4 points) | 8 (17.4%) | 5 (10.8%) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download