Abstract
This study aimed to delineate the possible impact of COVID-19 on acute myeloid leukemia (AML) patients in terms of diagnosis, chemotherapy, bone marrow transplant, and vaccination response. Allogeneic stem cell transplantation is markedly affected by the COVID-19 pandemic, as both donors and recipients must be healthy for transplantation to be feasible and successful. Delays in the identification of well-matched donors have been predicted, and represent a special challenge. Therefore, future donors should be tested for COVID-19. The outcome of delayed transplantation is vague and masked by variations in stem cell source along with disease subtype. However, if transplant delay results in recurrence of minimal residual disease, a negative impact on survival is anticipated.
AML patients are at high risk for coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) due to an immunocompromised state. Allogeneic stem cell transplant (SCT) remains the best treatment option for patients with intermediate- or high-risk AML. The COVID-19 pandemic presents great challenges for allogeneic-SCT. The clinical course of COVID-19 in patients with AML is unusual. Therefore, consistent polymerase chain reaction (PCR) and IgG antibody tests are mandatory for safely performing allogeneic-SCT. Constant hygiene measures may further help save patients during the COVID-19 pandemic [1].
As COVID-19 is a serious disease during an early pandemic, allogeneic-SCT in AML patients is believed to be at a particular risk for poor outcomes due to an immunocompromised state. Therefore, early diagnosis and supportive treatment may improve the prognosis of these patients. Close evaluation of stem cell donors and transplant candidates is also needed. Thus, continuous supportive treatment can lead to favorable outcomes, even in heavily immunocompromised patients [2].
Patients with AML are considered a high-risk population because of their immunocompromised status. Current studies focused on patients with AML have confirmed that COVID-19 severity tends to increase in immunocompromised patients. The presence of pulmonary infiltrates at diagnosis and the need for oxygen suggest poor outcomes. Therefore, social distancing, use of protective equipment, and infection control may be needed to save allogeneic-SCT patients [3].
Allogeneic-SCT patients can recover from COVID-19 and produce an antibody reaction with a similar overall survival to the general population. Poor prognosis is usually observed in AML patients with relapse and in those with risk factors, including comorbidities and neutropenia. Therefore, allogeneic-SCT patients should seek medical care, especially those with severe symptoms or complications [3].
Finally, COVID-19 infection results in high morbidity and mortality rates in allogeneic-SCT patients. Therefore, prevention of infection is essential. The most critical factors were old age and presence of comorbidities. Hopefully, centers can aid in protective measures, including vaccines, to decrease poor outcomes in allogeneic-SCT patients. This study aimed to show the impact of ongoing COVID-19 on patients with AML undergoing allogeneic-SCT [4].
The clinical course of COVID-19 in AML patients could be different from that in other COVID-19 cases. The incubation period for COVID-19 was approximately 14 days. However, the incubation period in AML patients is more than 3 weeks, irrespective of clear contact with a positive case. This finding could be explained by the fact that impaired immunity does not produce an immediate, strong reaction. The immune response is less potent than that in a healthy population. Intensive mouthwashes and chemotherapy might also be a possible explanation for the longer incubation periods in patients with AML. Therefore, consistent PCR and IgG antibody tests are necessary to confirm the diagnosis of COVID-19 in patients with AML undergoing allogeneic-SCT [5].
Respiratory and plasma specimens are collected regularly for SARS-CoV-2 PCR and antibody tests over the course of the disease. SARS-CoV-2 PCR, along with corresponding IgG antibody tests, is necessary. The SARS-CoV-2 IgG assay recognizes serum IgG antibodies directed against the nucleocapsid protein of the virus. SARS-CoV-2 is a relevant point during the early stage of the disease and is related to specific antibody reactions, revealing a relationship between the humoral immune response and control of SARS-CoV-2 [2].
The estimated seroconversion was approximately 10-14 days after diagnosis. However, SARS-CoV-2 antibodies have been detected in AML patients for more than 14 days due to an immunocompromised state. As immunosuppression and comorbidities are considered risk factors for COVID-19, delayed seroconversion can lead to negative outcomes for AML patients and for people with only restricted facilities of intermediate-care therapy [2].
At the time of COVID-19 diagnosis, 22 percent of AML patients had two comorbidities, including hypertension, congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, HIV, and chronic kidney disease, while 44 percent had none of these issues. Having more than 2 comorbidities with an active malignancy, the presence of pulmonary infiltrates on initial imaging, and neutropenia predicts increased disease severity in allogeneic-SCT patients [3].
Most recent recommendations for COVID-19 screening limit diagnostic tests to symptomatic, high-risk AML patients. Meanwhile, others are instructed to self-isolation/ quarantine. Full blood counts will be performed only in patients with AML with severe signs and confirmed COVID-19 infection. As 50–75% of AML patients are febrile at diagnosis, a missed or delayed diagnosis in these patients is suspected [6].
COVID-19 diagnosis may raise difficulties in the management of underlying leukemia, including delays in therapy and dose reduction. COVID-19 tests were routinely performed before induction therapy. In asymptomatic and symptomatic COVID-19 positive patients, induction therapy is postponed for 14 days, if possible. Delay in chemotherapy may adversely affect prognosis, particularly in young patients aged <60 years with favorable- or intermediate-risk diseases. For AML patients with favorable- or intermediate-risk who are fit to receive intensive chemotherapy, the standard “3+7” induction therapy should be considered. For consolidation, the cytarabine dose could be decreased to 1.5 g/m2 rather than 3 g/m2. Further therapy may be required to achieve deep complete remission before allogeneic-SCT [6-8].
The COVID-19 pandemic has created barriers for timely donor assessment, cell collection, and graft transport. Donors should practice good hygiene for approximately 14 days prior to donation. Therefore, unnecessary travel must be postponed. Recent guidelines suggest that donors should be tested for COVID-19 before cell collection. Stem cell products can be preserved at the collection site if transport is delayed. Cryopreservation of stem cell products is a safe procedure during COVID-19, preventing annoying events such as postponed transport or last-minute calls for an ineligible donor who is COVID-19 positive on the collection day, that could prevent graft delivery on time to an AML patient who has already received the conditioning regimen [9].
In patients with prior COVID-19, allogeneic-SCT is safe and possible; no recurrent COVID-19 is observed. However, persistent COVID-19 infection in AML patients highlights the potential risk of allogeneic-SCT. AML patients with severe complications after COVID-19 are unsuitable candidates for SCT. All patients had negative PCR results immediately prior to SCT. Therefore, allogeneic-SCT is possible 1–3 months after resolution of severe COVID-19. The dynamics of symptom improvement, negative PCR, CT-Chest, and pulmonary function tests are considered objective measures for the ideal time of allogeneic-SCT in patients with AML [1, 10].
The impact of COVID-19 on clinical outcomes of allogeneic-SCT was assessed at the Dana-Farber Cancer Center by assessing outcomes in 62 allogeneic-SCT recipients treated during the initial COVID-19 infection. Outcomes were compared with those of 66 allogeneic-SCT recipients treated before the pandemic. Despite structural changes during COVID-19, the 100-day overall survival, progression-free survival, primary graft failure, neutrophil engraftment, rates of non-COVID-19 infections, and acute graft-versus-host disease in allogeneic-SCT recipients were all similar during and before COVID-19 [11].
Overall survival (92% vs. 94%) and progression-free survival (83% vs. 85%) on day 100 were similar between allogeneic-SCT recipients during and before COVID-19. Donor product cryopreservation does not negatively affect early clinical outcomes. Despite cryopreservation, there was no difference in the incidence of primary graft failure (3.1% vs. 2.9%) or days to neutrophil engraftment (15 vs. 15 days) during and before COVID-19 [11].
The early relapse rates (9.4% vs. 11.8%) were similar in allogeneic-SCT recipients during and before COVID-19. Furthermore, there was no difference in the incidence of acute Graft versus host disease (GVHD) grade three to four (8.2% vs. 1.5%) during and before COVID-19. Cryopreservation is associated with reduced total leukocyte and CD3 chimerism. Total leukocyte chimerism (99% vs. 98%) and CD3 chimerism (95% vs. 67%) did not change significantly during and after COVID-19 [11].
There was also no significant difference in the incidence of non-COVID-19 and febrile neutropenia before and during COVID-19. This finding could be explained by the fact that most infections in allogeneic-SCT recipients are bloodstream (gut translocation) infections, that are not affected by a viral respiratory pandemic. Therefore, allogeneic-SCT recipients are regularly advised to adhere strictly to masks when they are immunosuppressed. There is no possible explanation for why there was no significant increase in non-COVID-19 infections in allogeneic-SCT recipients. Undetected COVID-19 is not expected, as the COVID-19 test is widely available for febrile patients [11].
Monitoring immune status after SCT is a standard clinical practice, involving lymphocyte subsets (CD4+ T cells, CD8+ T cells, CD19+ B cells, CD56+CD16+ NK cells, and CD3+ CD56+CD16+ NKT cells). Furthermore, T-cell populations include naive (CD45RA+CCR7+), central memory (CD45RA–CCR7+), and effector memory (CD45RA–CCR7–). Fig. 1 shows the immune subset study performed on allogeneic-SCT recipients [3].
COVID-19 is associated with a decrease in lymphocyte subsets. As allogeneic-SCT recipients are a special population, immune cells are affected by immune/disease status, immunosuppression regimen, and GVHD. Immunological profiling is commonly performed before COVID-19 diagnosis [3].
Most patients with AML have decreased lymphocyte subsets, particularly CD4 and CD8 T cells; B cells and NK cells in several patients have remained stable or slightly increased. The CD4 to CD8 ratio differs among patients with AML, with the likelihood of raising CD4 T cells. Within 2 years of allogeneic-SCT, lymphocyte subsets were compared during and before COVID-19 and revealed how COVID-19 is associated with lower lymphocyte counts, particularly T cells [3].
Lymphopenia with COVID-19 does not impair the immune status in allogeneic-SCT recipients; despite lymphopenia, AML patients can produce IgG antibodies against COVID-19. Because COVID-19 tests are widely available, 38 patients underwent antibody tests at a median of 37 days after diagnosis. Sixty-six percent of the patients developed antibodies, including 5 of 10 patients on immunosuppressive therapy. For patients with antibodies, it is thought to be a truly positive response if repeated tests have remained positive 1 week later [3]. Fig. 1 shows the assessment of lymphocyte subsets before and during recovery from COVID-19 in allogeneic-SCT recipients [3].
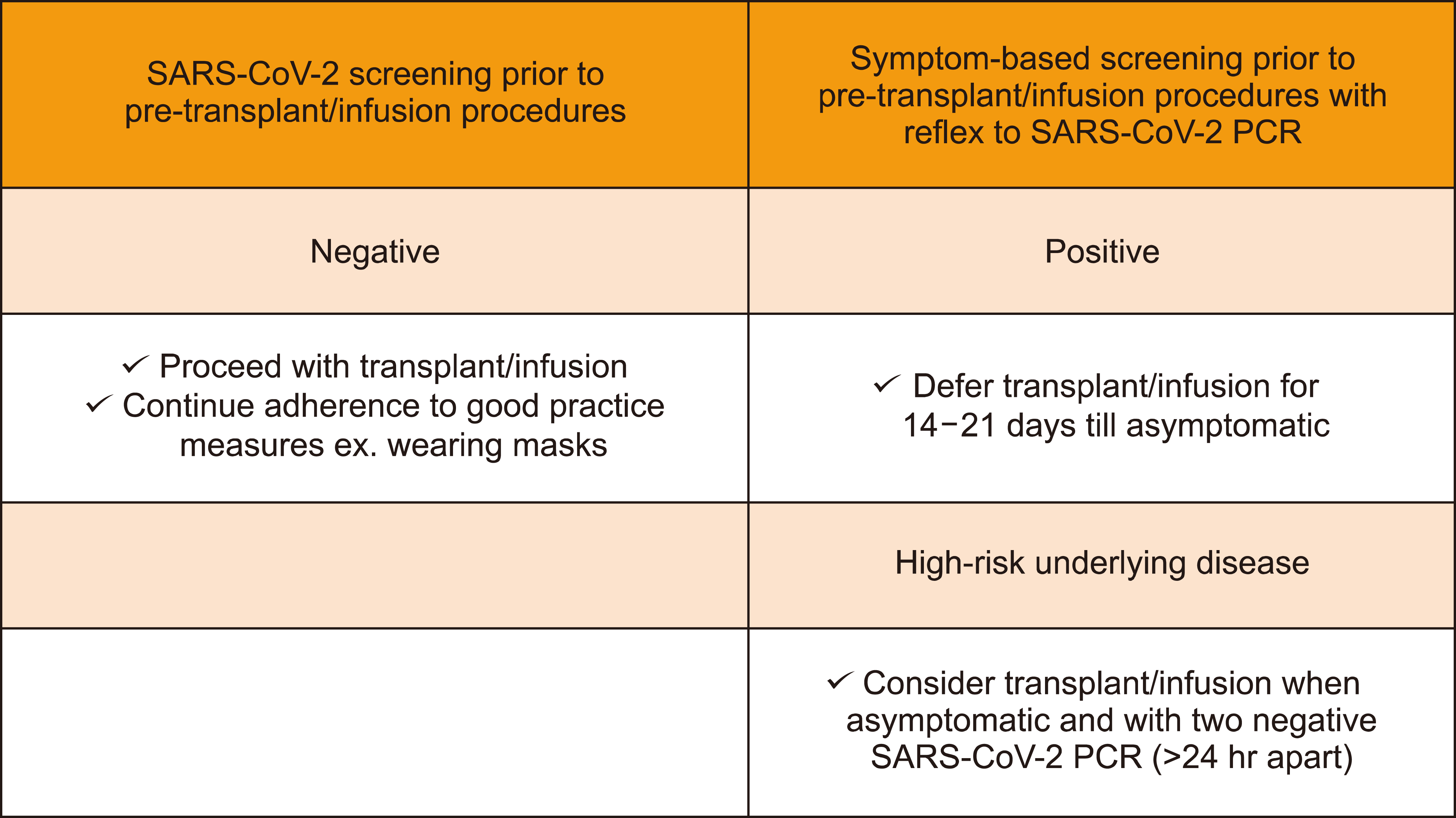
SARS-COV-2 screening before allogeneic-SCT in patients with AML should be performed. If the screening result is negative, transplantation should be started with appropriate precautions. However, if symptomatic patients are SARS-COV-2 positive, allogeneic-SCT should be postponed until improvement of symptoms or negative PCR results (Fig. 2) [12].
In patients with high-risk malignancies, transplantation procedures involving peripheral stem cell mobilization, bone marrow cell gathering, T cell collection, and conditioning regimens should be postponed for at least 14 days until the patient is asymptomatic and has at least two successive negative PCR tests 24 hours apart (Fig. 2) [12].
In patients with SARS-CoV-2 exposure, transplantation procedures involving peripheral stem cell mobilization, bone marrow cell gathering, T cell collection, and conditioning regimens should be postponed for 14 days from the date of the last contact. Exposed patients should be closely evaluated for the development of symptoms, and if asymptomatic, they should have two successive negative PCR tests 24 h apart before proceeding with the transplant [12].
Finally, allogeneic-SCT recipients should undergo screening for SARS-CoV-2 infection by PCR for at least 3 days before the transplantation procedures and conditioning regimens. Allogeneic-SCT recipients should avoid unnecessary travel, maintain appropriate hygiene, social isolation, and wear a mask in public [12].
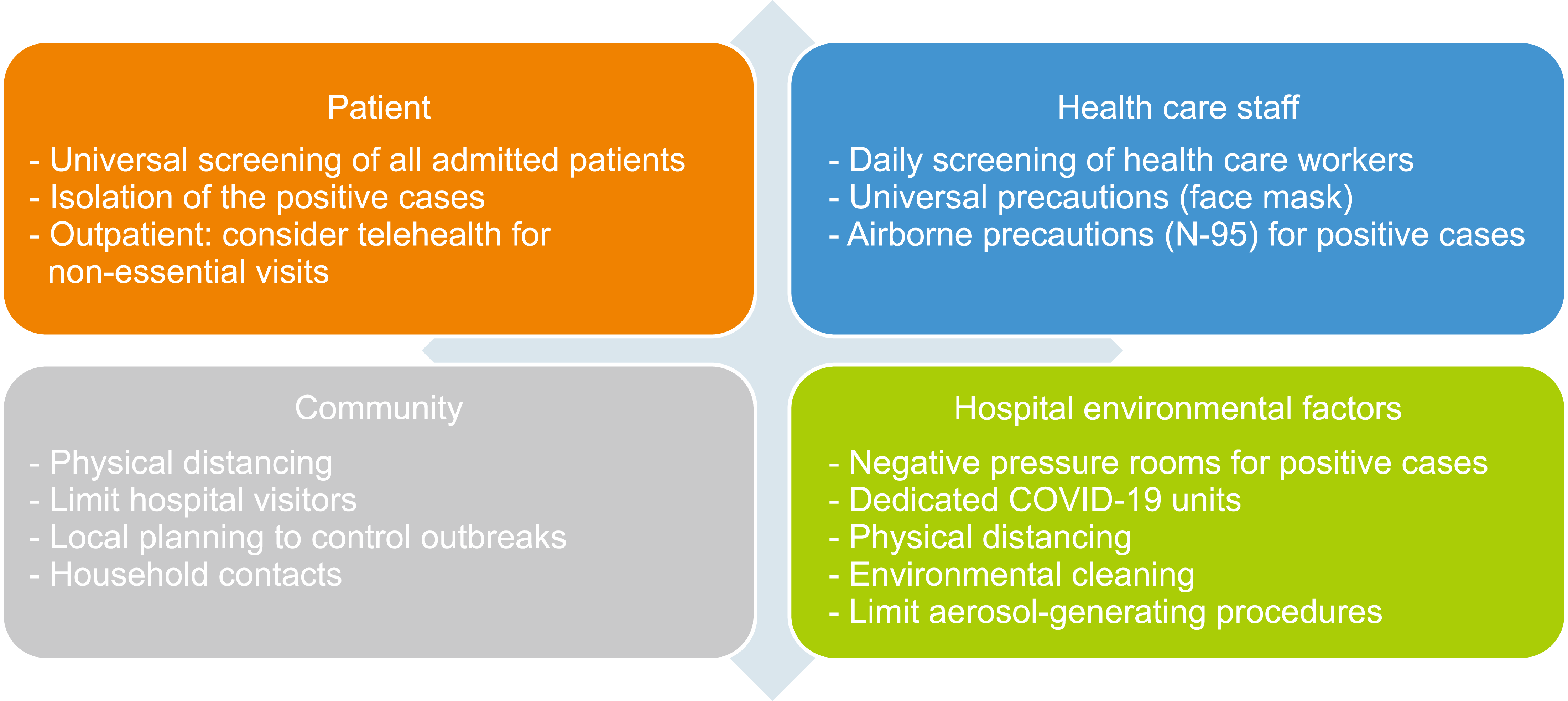
National guidelines have developed infection control measures for COVID-19. Allogeneic-SCT recipients are special populations that are at a high risk of developing COVID-19. Significant efforts have been made to prevent infection transmission, including the proper use of protective equipment among staff, symptom screening, and early isolation of symptomatic individuals [12].
Universal screening of symptomatic or exposed patients for a known COVID-19 case should be performed. Early isolation of symptomatic patients and screening for SARS-CoV-2 using polymerase chain reaction (PCR) are mandatory. Universal mask-wearing is essential in patients. Restricted appointments and telehealth services should be applied to outpatients. Education on COVID-19 prevention should also be provided to patients (Fig. 3) [12].
The prevention of self-contamination while wearing masks, disinfection of face shields between patients, and proper use of masks/eyewear are highly recommended for all staff. Further, all staff should perform SARS-CoV-2 screening, and symptomatic staff should be isolated. Asymptomatic staff tests are not recommended unless the patient develops symptoms or is in contact with a positive case (Fig. 3) [12].
Universal screening of symptomatic or exposed caregivers for a known COVID-19 case should be performed. Universal mask wearing by caregivers should be applied. Limiting the number of visitors and prohibiting visitors aged <12 years are very important. Caregivers should also provide education on COVID-19 prevention [12].
Transplant units should take additional measures to prevent respiratory pathogen transmission and fungal infections, involving isolated rooms for high-risk patients and highly effective air systems. Furthermore, clinical spaces, workrooms, and hospital centers are not ideal for social distancing/isolation (Fig. 3) [12].
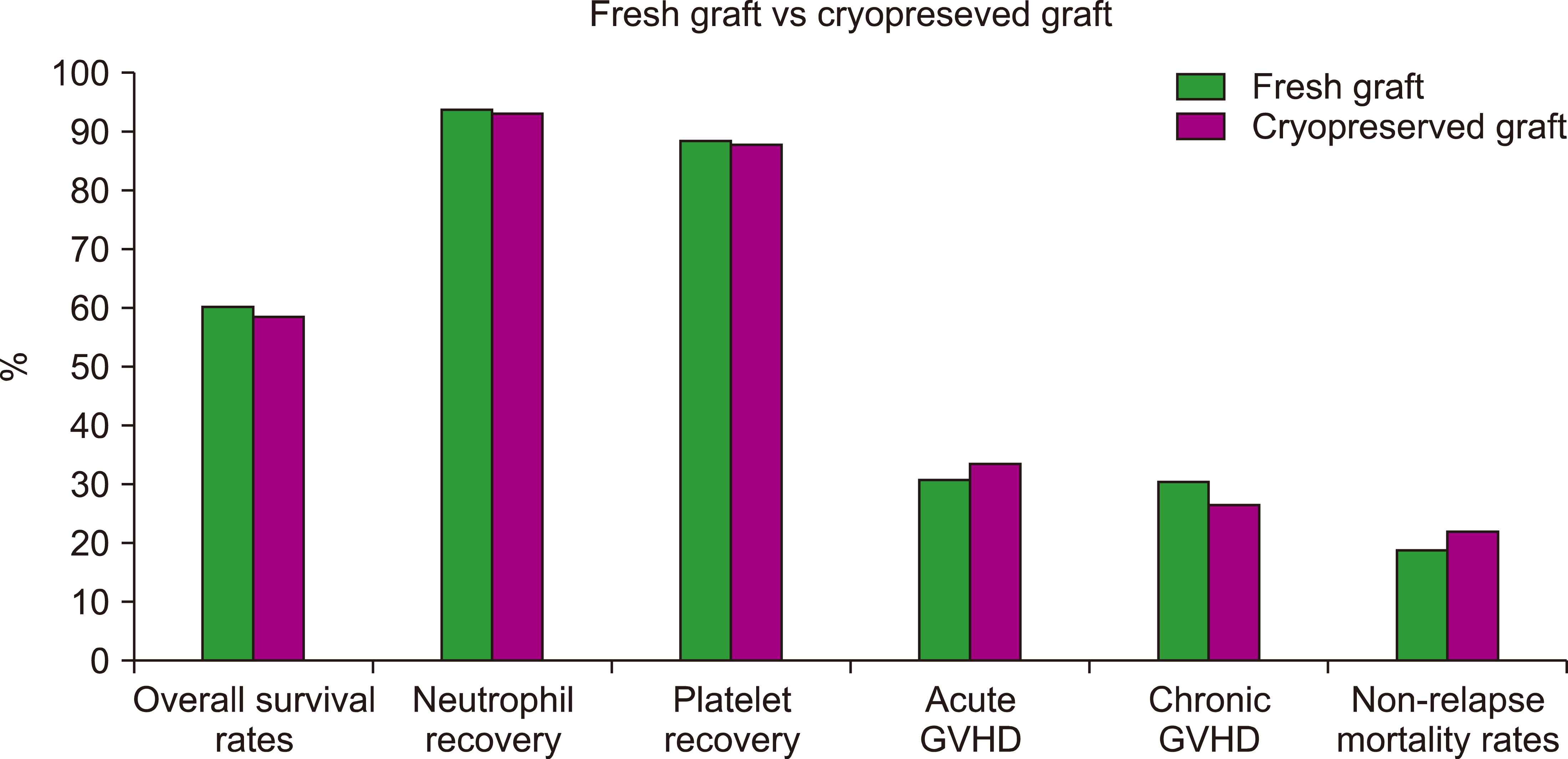
Among the 1,354 AML patients, 1,080 received fresh grafts and 274 received cryopreserved grafts. The median follow-up period was 24 months for fresh grafts and 23 months for cryopreserved grafts. In this study, patients have done allogeneic-SCT and received post-transplantation cyclophosphamide conditioning for GVHD prophylaxis. Patients with AML in the first or second complete remission were included in the study. Two-year overall survival rates were 60.6% in the fresh graft and 58.7% in the cryopreserved grafts (Fig. 4). Graft cryopreservation was not associated with a significantly high mortality rate [13].
The incidences of neutrophil recovery were 93.8% in fresh grafts and 93.3%, cryopreserved grafts. The median times to neutrophil recovery was 16 and 17 days, respectively. The incidences of platelet recovery were 88.8% and 87.7%. The median times for platelet recovery were 24 and 26 days, respectively. Graft cryopreservation was not associated with delayed recovery of neutrophils or platelets [13].
The incidences of acute GVHD were 31.3% for fresh grafts and 34% for cryopreserved grafts. The corresponding rates of acute GVHD were 9.4% and 6.3%. The incidences of chronic GVHD were 30.7% and 26.8%. Graft cryopreservation is associated with a lower risk of chronic GVHD [13].
The 2-year non-relapse mortality rate was 19.0% for fresh grafts and 22.0% for cryopreserved grafts. The corresponding rates of relapse/progression were 30.7% and 36.3% and the corresponding rates of disease-free survival were 50.4% and 41.7% [13].
Finally, cryopreserved grafts were not associated with a significantly higher mortality rate. Overall survival rates were 71.1% for fresh grafts and 70.3% for cryopreserved grafts. There was no evidence of delayed hematopoietic recovery or higher rates of acute or chronic GVHD with cryopreservation in allogeneic-SCT recipients. In conclusion, cryopreservation of donor allografts appears to be safe and suitable for patients with AML during the ongoing COVID-19 pandemic [13].
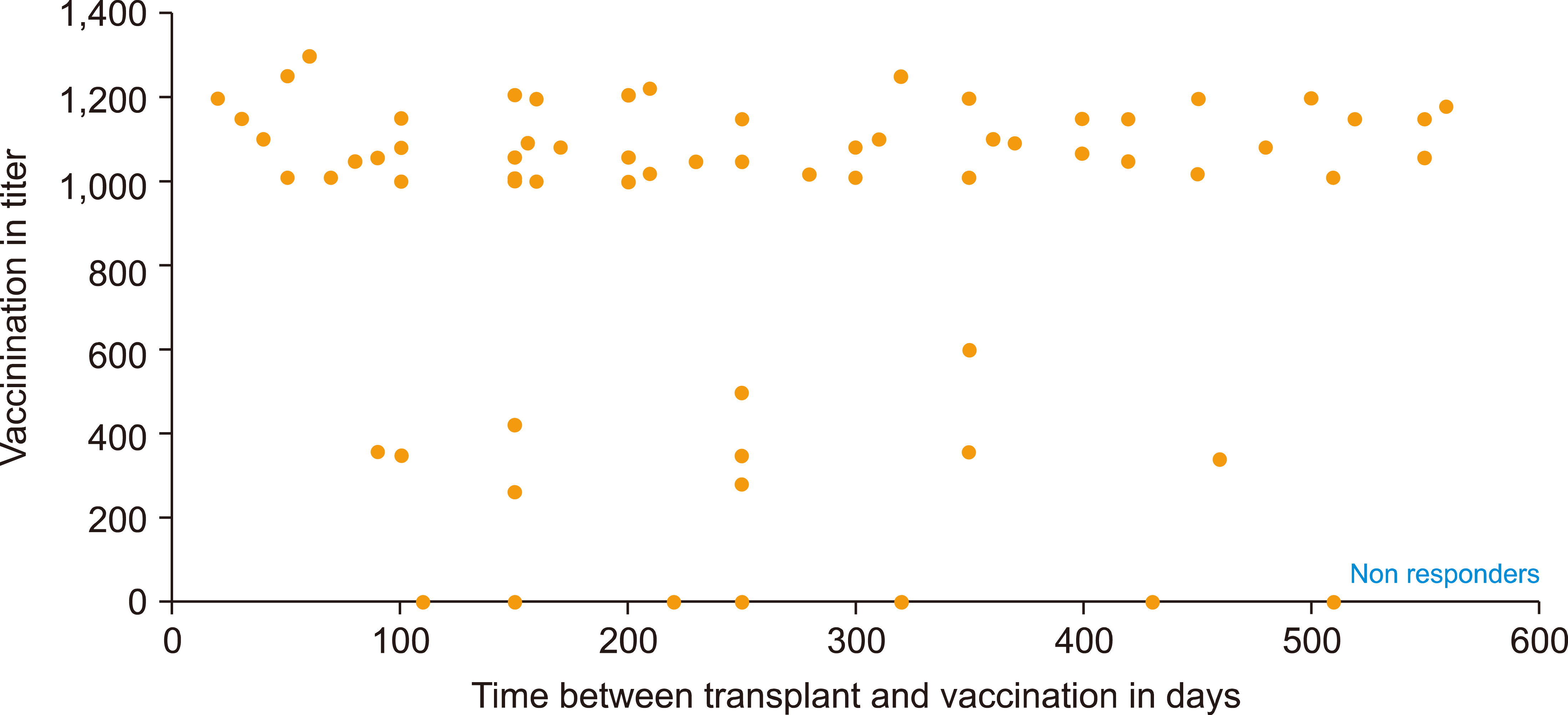
Seventy AML patients received post-transplantation cyclophosphamide conditioning (PTC) after SCT. These patients had received the COVID-19 vaccine. The antibody responses were determined using the SARS-CoV-2 IgG EUA assay. A cut-off level of 300 arbitrary units (AU)/mL level was chosen, and responders were categorized into sufficient/adequate responders (>300 BAU/mL) and low/non-responders [14].
Among 70 successive AML patients, 54 received Moderna, 8 received Pfizer, 6 received both, and 2 received Astra Zeneca. The median time between allogeneic-SCT and the first vaccination was around 27.8 months [14].
Of those patients, 61 developed sufficient/adequate responses (titer >300 AU/mL), and 53 had vigorous responses (titer >1,000 AU/mL). Nine patients could not develop a sufficient response; 2 patients were low responders (titer 0 ≤300 AU/mL) and 7 patients were non-responders (Fig. 5) [14].
Of the seven non-responders, four received GVHD prophylaxis (one patient received ibrutinib, one received tacrolimus, and two received ruxolitinib). Of those patients with sufficient/adequate responses, 10 received tacrolimus, while patients using ruxolitinib or ibrutinib were non-responders [14].
Therefore, Allogeneic-SCT recipients with COVID-19 should be vaccinated. Patients with AML who obtained PTC have a prolonged antibody reaction (>5 months) after vaccination. Vaccination after allogeneic-SCT with tacrolimus resulted in adequate antibody reactions (Fig. 5) [14].
The general ECIL guidelines recommend vaccination 3 months after SCT to induce an adequate response. Therefore, allogeneic-SCT recipients who received PTC showed a vigorous immune response to the vaccine, even when received early after SCT, while using tacrolimus [14].
COVID-19 has established barriers to timely donor evaluation, cell collection, and graft transport in allogeneic-SCT recipients. The availability of donor cells on the scheduled infusion date is extremely difficult during this period. Thus, patients with AML are at a higher risk of severe complications due to their immunocompromised state. Missed diagnosis, delay in chemotherapy, and transplantation may occur because of a lack of isolation beds and blood products during COVID-19. Finally, this study suggests the impact of ongoing COVID-19 on AML patients undergoing allogeneic-SCT.
ACKNOWLEDGMENTS
I am grateful for completing this wonderful article. Thanks to Allah for giving me the ability to complete this task. Thank you very much for all my relatives and friends who helped me. I appreciate Dr. Shaimaa El-Ashwah and Dr. May Denewer who helped me in finalizing this research within the limited time frame.
REFERENCES
1. Shah N, Dahi PB, Ponce DM, et al. 2022; Hematopoietic cell trans-plantation is feasible in patients with prior COVID-19 infection. Transplant Cell Ther. 28:55e1–5. DOI: 10.1016/j.jtct.2021.10.004. PMID: 34649021. PMCID: PMC8503970.

2. Di Cristanziano V, Meyer-Schwickerath C, Eberhardt KA, et al. 2021; Detection of SARS-CoV-2 viremia before onset of COVID-19 symptoms in an allo-transplanted patient with acute leukemia. Bone Marrow Transplant. 56:716–9. DOI: 10.1038/s41409-020-01059-y. PMID: 32943755.

3. Shah GL, DeWolf S, Lee YJ, et al. 2020; Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 130:6656–67. DOI: 10.1172/JCI141777. PMID: 32897885. PMCID: PMC7685738.

4. Schaffrath J, Brummer C, Wolff D, et al. 2022; High mortality of COVID-19 early after allogeneic stem cell transplantation: a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Transplant Cell Ther. 28:337e1–10. DOI: 10.1016/j.jtct.2022.03.010. PMID: 35296445. PMCID: PMC8918088.

5. Greiner J, Götz M, Malner-Wagner W, et al. 2021; Characteristics and mechanisms to control a COVID‐19 outbreak on a leukemia and stem cell transplantation unit. Cancer Med. 10:237–46. DOI: 10.1002/cam4.3612. PMID: 33314627. PMCID: PMC7826490.

6. Gavillet M, Carr Klappert J, Spertini O, Blum S. 2020; Acute leukemia in the time of COVID-19. Leuk Res. 92:106353. DOI: 10.1016/j.leukres.2020.106353. PMID: 32251934. PMCID: PMC7138175.

7. Raza A, Assal A, Ali AM, Jurcic JG. 2020; Rewriting the rules for care of MDS and AML patients in the time of COVID-19. Leuk Res Rep. 13:100201. DOI: 10.1016/j.lrr.2020.100201. PMID: 32318330. PMCID: PMC7169905.

8. Brissot E, Labopin M, Baron F, et al. 2021; Management of patients with acute leukemia during the COVID-19 outbreak: practical guidelines from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 56:532–5. DOI: 10.1038/s41409-020-0970-x. PMID: 32528122. PMCID: PMC7289070.

9. Leclerc M, Fourati S, Menouche D, Challine D, Maury S. 2021; Allogeneic haematopoietic stem cell transplantation from SARS-CoV-2 positive donors. Lancet Haematol. 8:e167–9. DOI: 10.1016/S2352-3026(21)00025-9. PMID: 33539769. PMCID: PMC7906696.

10. Christopeit M, Reichard M, Niederwieser C, et al. 2021; Allogeneic stem cell transplantation in acute leukemia patients after COVID-19 infection. Bone Marrow Transplant. 56:1478–81. DOI: 10.1038/s41409-021-01225-w. PMID: 33564122. PMCID: PMC7871512.

11. Maurer K, Saucier A, Kim HT, et al. 2021; COVID-19 and hematopoietic stem cell transplantation and immune effector cell therapy: a US cancer center experience. Blood Adv. 5:861–71. DOI: 10.1182/bloodadvances.2020003883. PMID: 33560397. PMCID: PMC7869610.

12. Waghmare A, Abidi MZ, Boeckh M, et al. 2020; Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant. 26:1983–94. DOI: 10.1016/j.bbmt.2020.07.027. PMID: 32736007. PMCID: PMC7386267.

13. Hamadani M, Zhang MJ, Tang XY, et al. 2020; Graft cryopreservation does not impact overall survival after allogeneic hematopoietic cell transplantation using post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 26:1312–7. DOI: 10.1016/j.bbmt.2020.04.001. PMID: 32283185. PMCID: PMC7194895.

14. Morsink LM, van Doesum J, Choi G, et al. 2022; Robust COVID-19 vaccination response after allogeneic stem cell transplantation using post transplantation cyclophosphamide conditioning. Blood Cancer J. 12:6. DOI: 10.1038/s41408-021-00605-1. PMID: 35022420. PMCID: PMC8754065. PMID: a194596df02245308c9774ef6b8c0022.

Fig. 1
Assessment of lymphocyte subsets over time before, during, and in recovery from COVID-19 in allogeneic-SCT recipients [3].
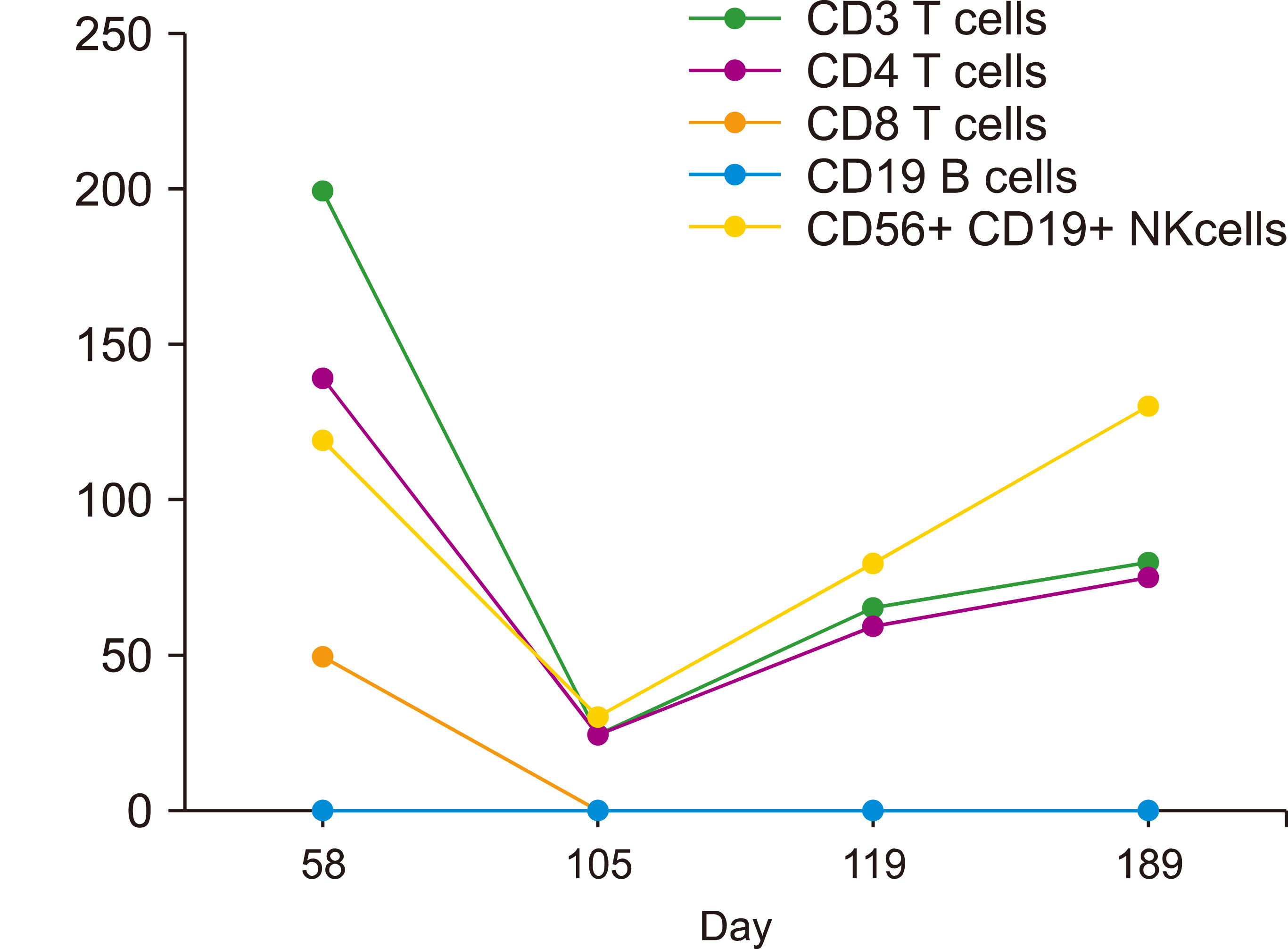
Fig. 5
Relationship between antibody titer after COVID-19 vaccination and time after allogeneic-SCT with post-transplantation cyclophos-phamide conditioning. Vaccination titer in AU/mL [14].




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download