INTRODUCTION
METHODS
1. Participants
2. Key variables
1) Serum vitamin D measurement
2) Depressive symptoms experience
3. Statistical analysis
RESULTS
1. Characteristics of the participants
Table 1.
|
Menopausal status |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pre-menopause (n=5,442) |
Post-menopause (n=6,131) |
||||||||||
| Q1 (n=1,365) | Q2 (n=1,359) | Q3 (n=1,359) | Q4 (n=1,359) | Pa | Q1 (n=1,534) | Q2 (n=1,533) | Q3 (n=1,538) | Q4 (n=1,526) | Pa | ||
| Mean vitamin D, mg/mL | 9.79±0.06 | 13.15±0.03 | 16.10±0.04 | 22.01±0.18 | <0.001 | 10.95±0.08 | 15.23±0.04 | 18.97±0.05 | 26.39±0.20 | <0.001 | |
| Age, y | 35.37±9.29 | 36.66±8.71 | 37.06±8.56 | 38.00±8.43 | 0.018 | 61.71±11.23 | 61.68±10.84 | 62.35±10.74 | 63.93±9.97 | 0.006 | |
| BMI | 0.038 | 0.005 | |||||||||
| ≥25 kg/m2 | 263 (19.27) | 291 (21.41) | 301 (22.15) | 306 (22.52) | 564 (36.77) | 624 (40.70) | 566 (36.80) | 511 (33.49) | |||
| Married (yes) | 855 (62.64) | 980 (72.11) | 1,018 (74.91) | 1,057 (77.78) | <0.001 | 1,101 (71.77) | 1,074 (70.06) | 1,107 (71.98) | 1,013 (66.38) | 0.297 | |
| Employed (yes) | 784 (57.44) | 755 (55.56) | 749 (55.11) | 730 (53.72) | 0.376 | 568 (37.03) | 619 (40.38) | 680 (44.21) | 671 (43.97) | 0.057 | |
| Area of residence | 0.503 | 0.135 | |||||||||
| Capital | 379 (27.77) | 411 (30.24) | 398 (29.29) | 414 (30.46) | 415 (27.05) | 374 (24.40) | 331 (21.52) | 334 (21.89) | |||
| Metropolitan | 300 (21.98) | 290 (21.34) | 266 (19.57) | 248 (18.25) | 304 (19.82) | 292 (19.05) | 308 (20.03) | 271 (17.76) | |||
| City/town | 686 (50.26) | 658 (48.42) | 695 (51.14) | 697 (51.29) | 815 (53.13) | 867 (56.56) | 899 (58.45) | 921 (60.35) | |||
| Household income | 0.764 | 0.137 | |||||||||
| Low | 323 (23.66) | 313 (23.03) | 292 (21.49) | 354 (26.05) | 369 (24.05) | 339 (22.11) | 393 (25.55) | 419 (27.46) | |||
| Mid-low | 352 (25.79) | 316 (23.25) | 348 (25.61) | 338 (24.87) | 396 (25.81) | 442 (28.83) | 369 (23.99) | 361 (23.66) | |||
| Mid-high | 319 (23.37) | 359 (26.42) | 364 (26.78) | 349 (25.68) | 400 (26.08) | 396 (25.83) | 381 (24.77) | 349 (22.87) | |||
| High | 371 (27.18) | 371 (27.30) | 355 (26.12) | 318 (23.40) | 369 (24.05) | 356 (23.22) | 395 (25.68) | 397 (26.02) | |||
| Education | 0.170 | <0.001 | |||||||||
| Elementary | 35 (2.56) | 18 (1.32) | 35 (2.58) | 50 (3.68) | 812 (52.93) | 819 (53.49) | 893 (58.06) | 934 (61.21) | |||
| Middle/high | 700 (5.128) | 653 (48.05) | 702 (51.66) | 704 (51.80) | 551 (35.92) | 592 (38.67) | 496 (32.25) | 474 (31.06) | |||
| University | 630 (46.15) | 688 (50.63) | 622 (45.77) | 605 (44.52) | 171 (11.15) | 120 (7.84) | 149 (9.69) | 118 (7.73) | |||
| METs | 0.003 | 0.003 | |||||||||
| Low | 601 (44.03) | 549 (40.40) | 506 (37.23) | 481 (35.39) | 747 (48.70) | 663 (43.25) | 620 (40.31) | 638 (41.81) | |||
| Moderate | 594 (43.52) | 596 (43.86) | 616 (45.33) | 616 (45.33) | 596 (38.85) | 620 (40.44) | 652 (42.39) | 607 (39.78) | |||
| High | 170 (12.45) | 214 (15.75) | 237 (17.44) | 262 (19.28) | 191 (12.45) | 250 (16.31) | 266 (17.30) | 281 (18.41) | |||
| Smoking | 0.334 | 0.056 | |||||||||
| Non-smoker | 1,178 (86.30) | 1,172 (86.24) | 1,185 (87.20) | 1,176 (86.53) | 1,433 (93.42) | 1,387 (90.48) | 1,435 (93.30) | 1,450 (95.02) | |||
| Past-smoker | 81 (5.93) | 99 (7.28) | 93 (6.84) | 100 (7.36) | 45 (2.93) | 69 (4.50) | 60 (3.90) | 40 (2.62) | |||
| Current-smoker | 106 (7.77) | 88 (6.48) | 81 (5.96) | 83 (6.11) | 56 (3.65) | 77 (5.02) | 43 (2.80) | 36 (2.36) | |||
| Alcohol, n/mon | 628 (46.24) | 684 (50.44) | 684 (50.78) | 693 (51.18) | 0.488 | 333 (21.74) | 420 (27.54) | 411 (26.78) | 373 (24.44) | 0.229 | |
| Comorbidity (yes) | 176 (12.89) | 170 (12.51) | 195 (14.35) | 216 (15.89) | 0.020 | 1,012 (65.97) | 988 (64.45) | 1,020 (66.32) | 1,113 (72.94) | 0.005 | |
| Stress perception | 0.222 | 0.898 | |||||||||
| Low | 904 (66.23) | 920 (67.70) | 949 (69.83) | 966 (71.08) | 1,154 (75.23) | 1,126 (73.45) | 1,142 (74.35) | 1,162 (76.30) | |||
| High | 461 (33.77) | 439 (32.30) | 410 (30.17) | 393 (28.92) | 380 (24.77) | 407 (26.55) | 394 (25.65) | 361 (23.70) | |||
| Dietary supplements (yes) | 457 (33.48) | 503 (37.07) | 571 (42.02) | 676 (49.74) | <0.001 | 644 (41.98) | 710 (46.31) | 777 (50.52) | 889 (58.26) | <0.001 | |
Values are presented as mean±standard deviation or number (%).
Vitamin D status was categorized into groups, according to serum 25(OH)D concentrations quartile. Premenopause: (Q1: 2.95-11.75 ng/mL, Q2: 11.76-14.55 ng/mL, Q3: 14.56-17.8 ng/mL, Q4: 17.81-44.59 ng/mL); postmenopause: (Q1: 4.11-13.39 ng/mL, Q2: 13.4-17.02 ng/mL, Q3: 17.02-21.29 ng/mL, Q4: 21.3-53.54 ng/mL).
Chronic diseases included hypertension, diabetes mellitus, dyslipidemia, stroke, cardiovascular disease, degenerative arthritis, rheumatoid arthritis, tuberculosis, asthma, thyroid disease, gastric cancer, liver cancer, colon cancer, breast cancer, cervicovaginal cancer, lung cancer, thyroid cancer, other cancer, chronic kidney disease, liver cirrhosis.
METs, practice vigorous activity of at least 75 min/wk, or moderate-intensity activity and/or walking of at least 150 min/wk, or any combination of walking, moderate-intensity or vigorous-intensity activities achieving at minimum of at least 600 metabolic equivalent-min/wk, according to low (<600), moderate (600-8,000), high (>8,000).
Abbreviations: 25-hydroxyvitamin D; BMI, body mass index; METs, metabolic equivalents; 25(OH)D, Q1, first quartile group; Q2, second quartile group; Q3, third quartile group; Q4, fourth quartile group.
2. Prevalence of depressive symptoms according to vitamin D quartiles
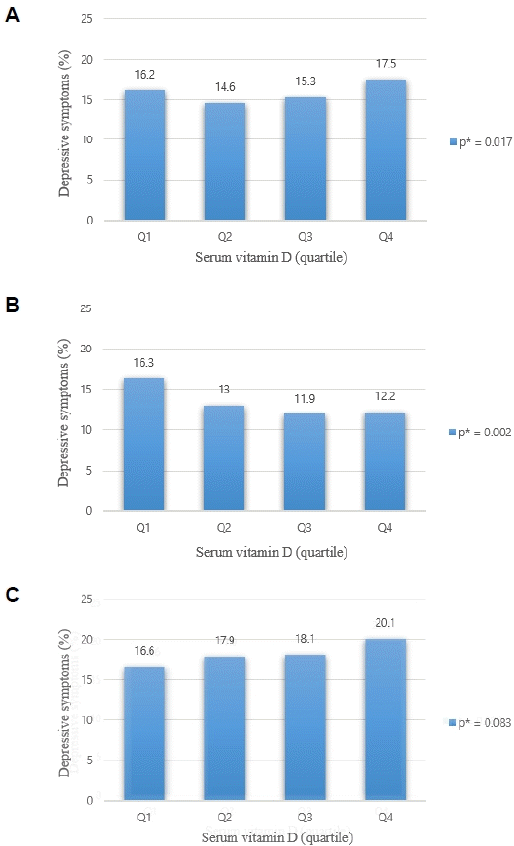 | Fig. 1.Association between vitamin D status and depression. (A) Total adult women, (B) premenopausal women, (C) postmenopausal women. Serum vitamin D status was categorized into groups, according to serum 25(OH)D concentrations quartile Total adult women: Q1: 2.95-12.48 ng/mL, Q2: 12.49-15.68 ng/mL, Q3: 15.69-19.7 ng/mL, Q4: 19.71-53.54 ng/mL. Premenopausal status: Q1: 2.95-11.75 ng/mL, Q2: 11.76-14.55 ng/mL, Q3: 14.56-17.8 ng/mL, Q4: 17.81-44.59 ng/mL. Postmenopausal status: Q1: 4.11-13.39 ng/mL, Q2: 13.4-17.02 ng/mL, Q3: 17.02-21.29 ng/mL, Q4: 21.3-53.54 ng/mL. *P-values were obtained using chi-square test. 25(OH)D, Q1, first quartile group; Q2, second quartile group; Q3, third quartile group; Q4, fourth quartile group; 25-hydroxyvitamin D. |
3. Multivariate analysis of depressive symptoms associated with vitamin D quartiles in pre- and post-menopausal women
Table 2.
|
Vitamin D status |
|||||
|---|---|---|---|---|---|
| Q1 (n=2,896) | Q2 (n=2,894) | Q3 (n=2,892) | Q4 (n=2,891) | Pa | |
| Depressive conditions | 468 | 423 | 443 | 507 | |
| Model 1 | 1 | 0.89 (0.71-1.12) | 0.87 (0.69-1.11) | 1.08 (0.88-1.31) | 0.112 |
| Model 2 | 1 | 0.83 (0.66-1.06) | 0.83 (0.64-1.07) | 0.99 (0.79-1.23) | 0.313 |
| Model 3 | 1 | 0.85 (0.67-1.08) | 0.84 (0.65-1.09) | 0.98 (0.78-1.22) | 0.371 |
Vaules ane presented as odds ratio (95% confidence interrml) or number.
Vitamin D status was categorized into groups, according to serum 25(OH)D concentrations quartile; female adults: Q1: 2.95-12.48 ng/mL, Q2: 12.49-15.68 ng/mL, Q3: 15.69-19.7 ng/mL, Q4: 19.71-53.54 ng/mL.
Model 1: unadjusted; model 2: adjustment for age, BMI, smoking, MET, drinking, comorbidity, stress, dietary supplements; model 3: additional adjustments for marriage, income, employment, education.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; MET, metabolic equivalent; Q1, first quartile group; Q2, second quartile group; Q3, third quartile group; Q4, fourth quartile group.
Table 3.
|
Vitamin D status |
Pa | ||||
|---|---|---|---|---|---|
| Q1 (n=1,365) | Q2 (n=1,359) | Q3 (n=1,359) | Q4 (n=1,359) | ||
| Depressive conditions | 223 | 177 | 162 | 166 | |
| Model 1 | 1 | 0.81 (0.59-1.11) | 0.73 (0.53-1.01) | 0.67 (0.48-0.94) | 0.018 |
| Model 2 | 1 | 0.73 (0.52-1.04) | 0.69 (0.49-0.98) | 0.64 (0.44-0.93) | 0.019 |
| Model 3 | 1 | 0.75 (0.53-1.07) | 0.70 (0.49-1.00) | 0.62 (0.43-0.92) | 0.016 |
Vaules ane presented as odds ratio (95% confidence interrml) or number.
Vitamin D status was categorized into groups, according to serum 25(OH)D concentrations quartile; premenopause: Q1: 2.95-11.75 ng/mL, Q2: 11.76-14.55 ng/mL, Q3: 14.56-17.8 ng/mL, Q4: 17.81-44.59 ng/mL.
Model 1: unadjusted; model 2: adjustment for age, BMI, smoking, MET, drinking, comorbidity, stress, dietary supplements; model 3: additional adjustments for marriage, income, employment, education.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; MET, metabolic equivalent; Q1, first quartile group; Q2, second quartile group; Q3, third quartile group; Q4, fourth quartile group.
Table 4.
|
Vitamin D status |
Pa | ||||
|---|---|---|---|---|---|
| Q1 (n=1,534) | Q2 (n=1,533) | Q3 (n=1,538) | Q4 (n=1,526) | ||
| Depressive conditions | 254 | 274 | 278 | 307 | |
| Model 1 | 1 | 1.08 (0.80-1.44) | 1.19 (0.89-1.60) | 1.27 (0.99-1.64) | 0.047 |
| Model 2 | 1 | 1.06 (0.78-1.43) | 1.17 (0.86-1.61) | 1.29 (0.99-1.67) | 0.048 |
| Model 3 | 1 | 1.06 (0.78-1.44) | 1.18 (0.87-1.61) | 1.27 (0.98-1.66) | 0.057 |
Vaules ane presented as odds ratio (95% confidence interrml) or number.
Vitamin D status was categorized into groups, according to serum 25(OH)D concentrations quartile; postmenopause: Q1: 4.11-13.39 ng/mL, Q2: 13.4-17.02 ng/mL, Q3: 17.02-21.29 ng/mL, Q4: 21.3-53.54 ng/mL.
Model 1: unadjusted; model 2: adjustment for age, BMI, smoking, MET, drinking, comorbidity, stress, dietary supplements; model 3: additional adjustments for marriage, income, employment, education.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; MET, metabolic equivalent; Q1, first quartile group; Q2, second quartile group; Q3, third quartile group; Q4, fourth quartile group.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download