Abstract
Background
The role of the sympathetic nervous system appears to be central in causing pain in complex regional pain syndrome (CRPS). The stellate ganglion block (SGB) using additives with local anesthetics is an established treatment modality. However, literature is sparse in support of selective benefits of different additives for SGB. Hence, the authors aimed to compare the efficacy and safety of clonidine with methylprednisolone as additives to ropivacaine in the SGB for treatment of CRPS.
Methods
A prospective randomized single blinded study (the investigator blinded to the study groups) was conducted among patients with CRPS-I of the upper limb, aged 18–70 years with American Society of Anaesthesiologists physical status I–III. Clonidine (15 μg) and methylprednisolone (40 mg) were compared as additives to 0.25% ropivacaine (5 mL) for SGB. After medical treatment for two weeks, patients in each of the two groups were given seven ultrasound guided SGBs on alternate days.
Results
There was no significant difference between the two groups with respect to visual analogue scale score, edema, or overall patient satisfaction. After 1.5 months follow-up, however, the group that received methylprednisolone had better improvement in range of motion. No significant side effects were seen with either drug.
Conclusions
The use of additives, both methylprednisolone and clonidine, is safe and effective for the SGB in CRPS. The significantly better improvement in joint mobility with methylprednisolone suggests that it should be considered promising as an additive to local anaesthetics when joint mobility is the concern.
Complex regional pain syndrome (CRPS) is a chronic pain disorder with significant neuropathic as well as autonomic features. It has a multifactorial pathogenesis that include peripheral and central sensitization, inflammation, altered sympathetic and catecholaminergic function, altered somatosensory representation in the brain, genetic factors and psychophysiological interactions [1]. With progressive evolution of the understanding of its etiopathogenesis, the role of the sympathetic nervous system appears to be central in causing the pain in CRPS [2]. Thus, a multidimensional approach, including sympathetic blocks, is aimed at controlling the pain and suffering.
Single shot stellate ganglion blocks (SGB) with local anaesthetics and different adjuvants are being tried at frequent interval for better pain relief [3]. Additives like dexamethasone, methylprednisolone, clonidine and ketamine have been studied and found to have positive results, though the mechanisms are different [4]. Clonidine (an α2 adrenergic agonist) has also shown promising result in CRPS treatment [5]. However, there is a paucity of literature stating the comparative advantages of specific adjuvants. Therefore, the current study was planned.
Hypothesizing that the addition of clonidine (15 μg) to ropivacaine (0.25%), would improve the onset, quality, and duration of pain relief as compared to methylprednisolone, as an additive in patients with CRPS I of the upper limb.
The literature survey for the study was done from related scientific journal articles and books using PubMed.
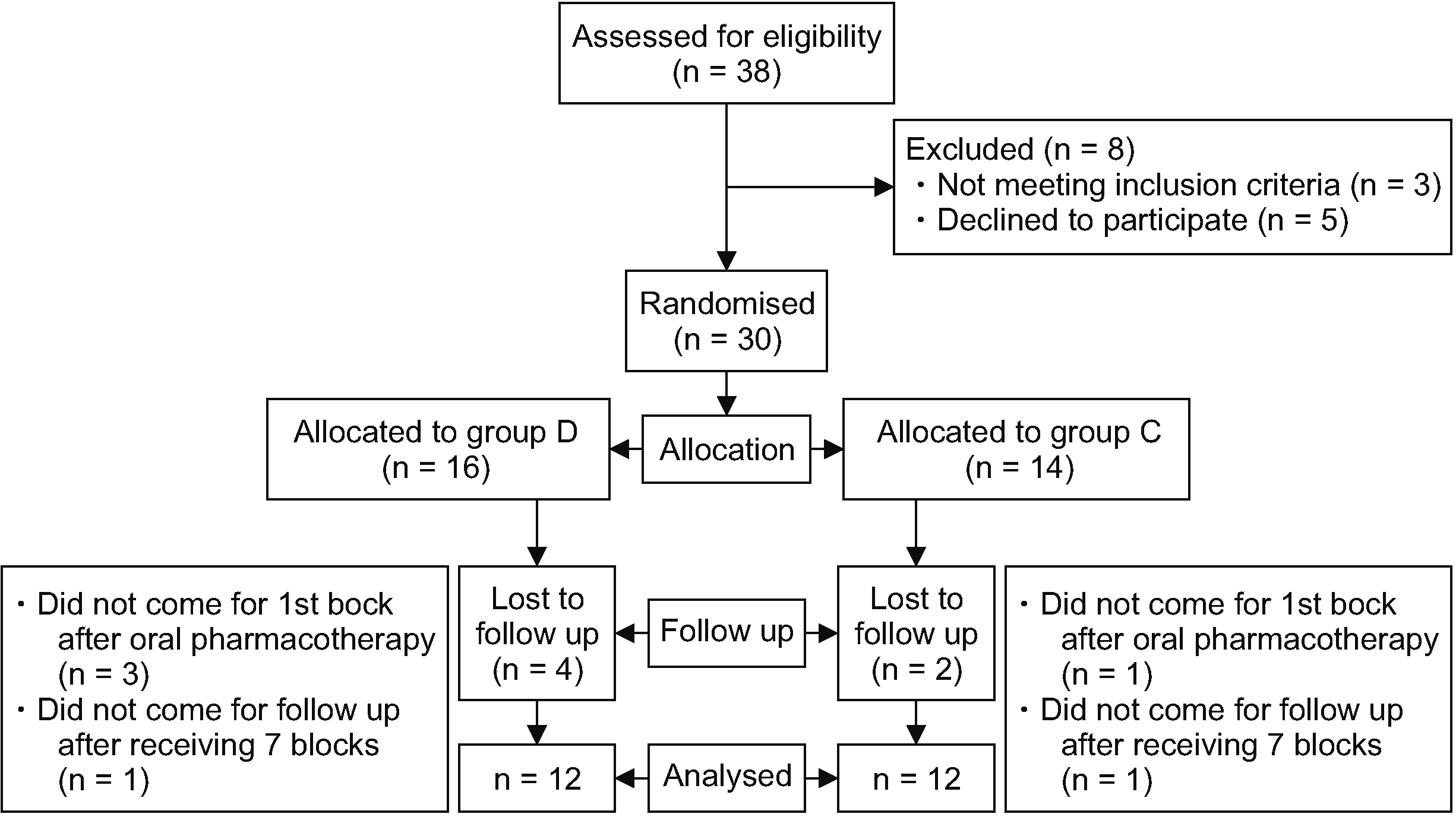
This study was conducted at the pain clinic of a tertiary care center after obtaining institutional ethical committee of All India Institute of Medical Sciences approval (IECPG-250/28.06.2018, RT-3/30/08/2018) and was then prospectively registered (CTRI number- CTRI/2019/01/017080). Written informed consent was obtained from all subjects. The study included patients with CRPS-I of upper limb, in the age group of 18–70 years with American Society of Anaesthesiologists (ASA) physical status I–III. Those having coagulation disorders, local infection at the puncture site, who were on beta blockers or alpha blockers, who had diabetic neuropathy, baseline heart rate less than or equal to 60/min, or drug or alcohol abuse were excluded. Thirty-two patients were randomly assigned to either of two groups based on computer generated random number tables. The control group (group D) received 40 mg methylprednisolone along with 0.25% ropivacaine (total volume 5 mL); and the study group (group C) received 15 μg clonidine along with 0.25% ropivacaine (total volume 5 mL). It was a single blinded study, where the investigator (blinded to the study groups) recorded the outcome parameters (Fig. 1).
Baseline severity of pain was measured using a visual analogue scale (VAS) on day 0. Pain at rest and the pain on movement/evoked pain was measured separately. The range of movement (ROM) of the joint was measured by the angles made with maximum effort at the different involved joints using a designated goniometer for that joint. The subject’s edema score was measured on a designated scale (Appendix 1). Assessment of the subjects’ quality of life was done by the Brief Pain Inventory (BPI) questionnaire (Appendix 2). Functional assessment was done using the Disabilities of Arm, Shoulder and Hand (Quick DASH) questionnaire (Appendix 3).
After obtaining informed and written consent, a standard medical treatment was given to all the patients that included oral amitriptyline (10 mg) at the hour of sleep (HS) as well as a combination of gabapentin (300 mg) and methylcobalamine (750 μg) at the HS for 1 week. If no adverse effect was noted then this was continued as a twice daily dose for one more week. The upper limit of the gabapentin dose was based upon past experience of most patients complaining of dizziness and becoming non-compliant when doses more than 600 mg/day were used; this is in agreement with the findings by Kamble et al. [6]. Also, oral aceclofenac (100 mg) and paracetamol (500 mg) combination twice daily was used, and a composite table containing tramadol (37.5 mg) and acetaminophen (325 mg) was prescribed when required. If a patient complained of gastric discomfort, ranitidine (150 mg) twice daily was prescribed as needed. After 2 weeks, the patients were assessed to see any change in their VAS scores, edema scores, and range of motion of involved joints.
Following this, the first block was given on the same day.
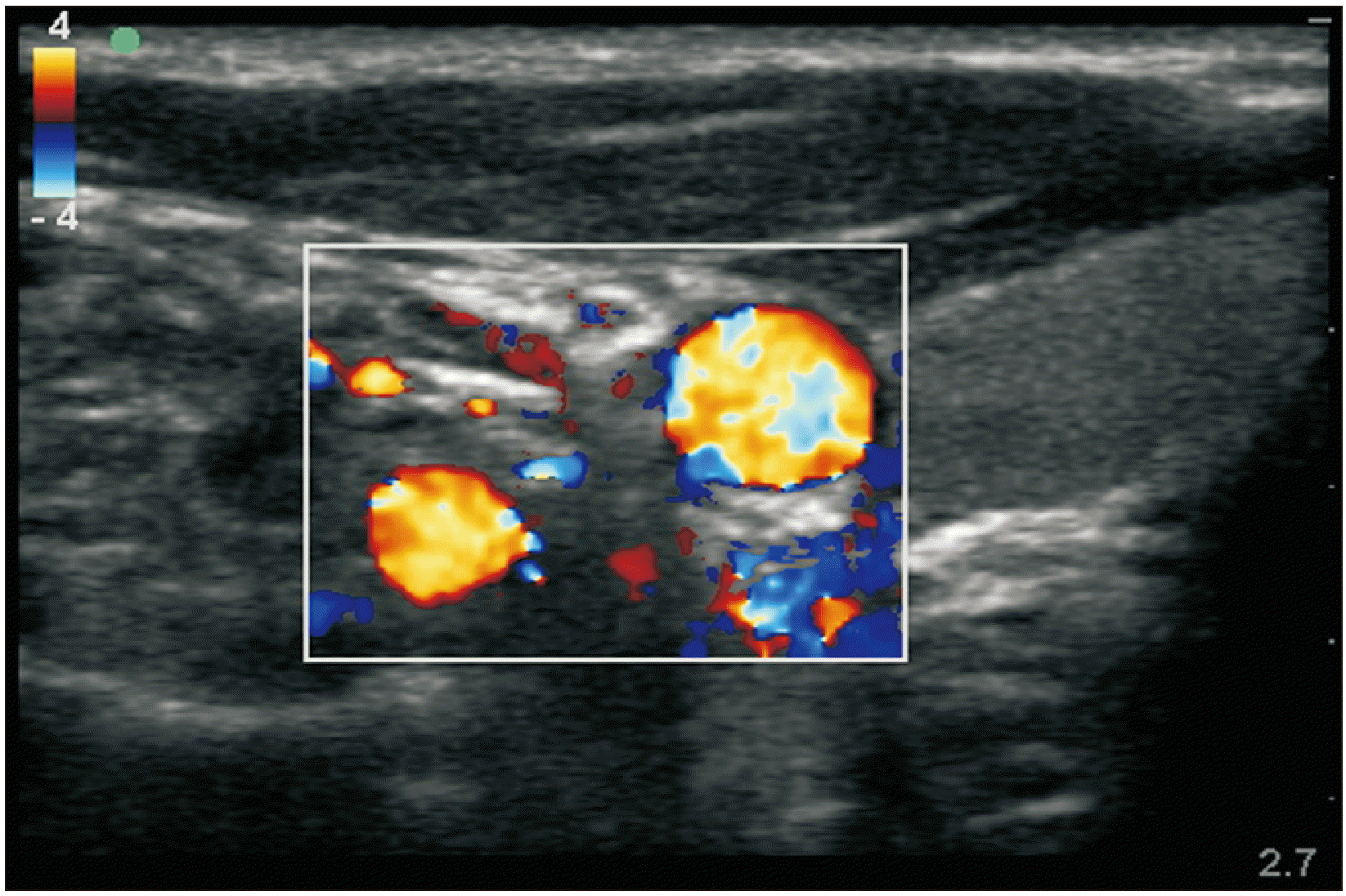
Patients were shifted to procedure room for performing the SGB under ultrasound (M-turbo; Sonosite) guidance. A 20/22-guage intravenous cannula was secured on the dorsum of the contralateral hand and IV fluids (Ringer lactate) started. Standard ASA monitoring was performed, and baseline parameters such as heart rate, blood pressure, SpO2, and the temperature of the ipsilateral hand skin using fever scan (infrared thermometer, Vandelay) were noted. The neck was turned to the opposite side and extended by slight elevation at the shoulder by placing a pillow underneath. A scout scan was performed using a high frequency linear probe (6–13 MHz) on the ventrolateral side of neck to visualize vital structures (Figs. 2, 3). Under strict aseptic measures, the skin puncture site was infiltrated with 1.5–2 mL of 2% lignocaine followed by insertion of a 22 G echogenic needle according to the technique described in the pilot study by Kapral et al. [7] (Fig. 4). Studies by Matsumoto [8,9] and Malmqvist et al. [10] have shown that blockade at the level of the C6 vertebra produced a more successful sympathetic blockade to the head and neck with less success with a sympathetic blockade to the upper extremities. However, sympathetic blockade at the level of the C7 vertebra produces a successful sympathetic blockade of the upper extremity [11]. Elias et al. [12] also made the same observation.
Patients in the control group received a series of ultrasound guided SGBs with 5 mL of 0.25% ropivacaine with 40 mg methylprednisolone as an additive; those in the study group received the block with 5 mL of 0.25% ropivacaine along with 15 μg clonidine. Development of ipsilateral Horner’s syndrome within 10 minutes of the first injection, along with relief of pain as experienced by the patient immediately and rise of the skin temperature of the ipsilateral hand were taken as the primary end points.
A separate blinded investigator monitored the patients during and after giving the block at 5, 15, and 30 minutes, for the parameters: heart rate, electrocardiogram, non-invasive blood pressure, pulse oximetry, and skin temperature of the ipsilateral side. The person injecting the drug did not participate in the recording of the parameters.
The skin surface temperature of the distal part of the involved upper extremities pre-and post-block was measured using a fever scanner (infrared thermometer from Vandelay).
Patients were shifted to the postanaesthesia care unit (PACU) for observation and documentation of any complication. Patients were discharged home after they met the standard PACU discharge criteria. They were asked to come for the next block after a gap of one day (i.e., alternate day block) and advised to continue physiotherapy. All patients continued their medications and physical therapy throughout the course of their treatment.
Subsequent blocks using the same technique and pain specialist were performed on every alternate day with 7 blocks for each patient, over a period of 2 weeks. Additionally, at the end of the SGB course (on the day of the last block) the parameters noted were: pain intensity using the VAS with and without movement, the edema score, ROM, BPI scores, Quick DASH score, and patient satisfaction score (Appendix 4).
Patients were followed up in the pain clinic at day 45 and 75. At each follow-up, the VAS scores, the ability to exercise, oral drug requirement, edema score, ROM of the joint, BPI scores, Quick DASH score, and patient satisfaction scores were recorded.
All the data were entered in a Microsoft Excel spreadsheet and analysis was done using Stat14 software. Categorical variables were presented in frequency (%) and continuous variables were presented as mean ± standard deviation or median (min/max). Normality of data was tested using the Shapiro–Wilk test. If the normality was rejected then a non-parametric test was used. Continuous variables following normal distribution were compared using Student’s t-test. Variables not following normal distribution were compared using the Wilcoxon rank sum test. Categorical variables were correlated using chi-square test/Fisher exact test. A P value of < 0.05 was considered statistically significant.
The required sample size was calculated using G*Power version 3.1.9.7 software (Heinrich Heine Universität). The primary outcome was the difference between the two groups with respect to the pain score, assessed on the VAS. It was estimated that a sample size of 14 patients per group would achieve a power of 79% to detect a large effect size (Cohen’s d) of 1.1. The statistical test used was the two-sided unpaired t-test and the type I error was set at 0.05. A total of 32 patients were randomly assigned.
A total of 32 patients were enrolled for the study and two were lost to follow up (CONSORT). The final study population was 30 patients, 14 in the study group and 16 in the control group.
Demographically, both the groups were statistically similar (Table 1). The mean age in group C was 51.7 ± 11.7 years (range 22 to 62) and in group D was 45.8 ± 12.8 years (range 25 to 61). In the group C, 7 (50.0%) patients were male and 7 (50.0%) were female. In the group D, 7 (43.8%) patients were male and 9 (56.2%) were female. In group C, all 14 patients were ASA grade I. In group D, one patient was hypertensive and hypothyroid on medications and belonged to ASA grade II, rest 15 were ASA grade I.
In the present study, the median resting VAS score for the wrist joint in group C improved from 9 (8–9) to 4 (3.25–4) at the completion of the SGB course. In group D, it showed an improvement from 9 (9–10) to 3 (3–4) (Table 2).
In patients with small joint (metacarpophalangeal and interphalangeal) involvement, the median resting VAS score in group C improved from 8 (8–9) to 3 (2.5–4) after completion of SGB course (Table 3). In group D, it showed an improvement from 9 (8–9) to 3.5 (3–4) after serial SGBs and after 2 weeks follow-up it was 3 (2.25–3.75).
All the patients in both the groups were subjected to the same physiotherapy, using hand exercises throughout the period of treatment, and follow up. In this study, although all patients showed a significant clinical improvement in VAS scores after receiving the treatment, but statistically there was no significant difference between the two groups at any point of time.
The ROM showed an appreciable clinical improvement in all the patients of both groups. However, for those with wrist joint involvement, there was a statistically significant difference in range of wrist extension between the two groups both at presentation and after 2 weeks of oral pharmacotherapy (P = 0.018) (Table 4). There was a statistically significant difference in the range of radial deviation of the wrist joint both after receiving 2 weeks of oral pharmacotherapy (P = 0.014) and at the end of the completion of all blocks and physiotherapy (P = 0.049) (Table 5). The group that received methylprednisolone (group D) had a better ROM than group C patients, who received clonidine as an additive, for both the joint movements mentioned. Although the ROM at the small joints of the hand improved clinically in both the groups, statistically there was no significant difference between the two groups (P = 0.504).
The edema score at the wrist joints (Fig. 5) and the small joints in both groups C and D showed appreciable clinical improvement, but the difference between the two groups was not significant statistically. In group C, 83.3% subjects had an edema score of 2 and 16.7% had an edema score of 1 at presentation; this reduced to 70% subjects with an edema score of 1 and 30.0% with an edema score of 0 at the end. In group D, 66.7% subjects had an edema score of 2 and 33.3% had an edema score of 1 at presentation; it reduced to 72.7% subjects with an edema score of 1 and 27.3% with an edema score of 0 at the end.
The study showed improvement in Quick DASH scores for both groups C and D, which was statistically comparable between the two groups at all points of time (Fig. 6). In group C, it improved from 66.4 ± 9.0 to 31.7 ± 10.3; and in group D, from 65.3 ± 16.4 to 33.5 ± 12.2.
Similarly, the BPI scores showed an improvement, which was also statistically comparable between the two groups (Fig. 7).
At the end of entire treatment course, the patient satisfaction scores for groups C and D were 7.583 ± 2.020 and 7.583 ± 1.240 respectively, which correspond to moderate to full patient satisfaction (Fig. 8). However, the difference between the two groups was not significant statistically.
There was no significant difference statistically in the haemodynamic parameters between the two groups during and after the SGB.
The mean rise in skin temperature over the affected hand after each block varied between 1.3°C to 2°C in group C and 1.4°C to 1.9°C in group D. Both the groups showed a rise in skin temperature after each block but the difference in skin temperature after the second block was more in group C compared to group D, and this was statistically significant (P = 0.036).
In the present study, all the patients developed Horner’s syndrome following each SGB. One patient in group D developed asymptomatic sinus bradycardia with a heart rate that reduced up to 45 bpm after receiving the 5th block. This persisted for 1 minute during which the patient was asymptomatic and blood pressure remained within normal limits. After 30 minutes of monitoring haemodynamic parameters, a 12 lead electrocardiogram (ECG) was done to rule out any significant changes, and it was normal. One patient in group C experienced slight drowsiness after each block, lasting for a period of 1–2 hours. The patient was kept under observation until he was comfortable to stand and walk without any difficulty.
The present study compared methylprednisolone and clonidine as additives to ropivacaine in the SGB for CRPS I treatment. It demonstrated equal clinical benefit and patient satisfaction from both, with better improvement in ROM with methylprednisolone.
There is little evidence in the literature to guide CRPS treatment, especially for patients where non-interventional treatment modalities do not help. Understanding its complex aetiopathogenesis has helped in planning treatment. The nociceptive afferent inputs cause hyperactive spinal neuron activities, which stimulate the sympathetic neurons to induce arterial spasms, ischemia, and edema [13]. This culminates into a typical symptomatology of disproportionate pain, swelling, skin and temperature changes, and restriction of joint mobility.
Among the interventional treatment methods, sympatholysis attempts to target CRPS symptomatology effectively. A sympathetic blockade has been recommended as early as possible to interrupt and reverse the process of CRPS [14].
The stellate ganglion, also known as the cervicothoracic ganglion, is a part of the cervical sympathetic chain. It provides sympathetic input to the ipsilateral upper extremity, chest, face, and head. A SGB has long been established as a treatment modality for CRPS, due to the ability to abolish the sympathetic response of the upper limb [3] and hence break the pain cycle.
Clonidine, a α2-adrenoceptor agonist, has been used for many years as an additive to short, intermediate, and long-acting local anesthetics for peripheral nerve blocks with significant analgesic benefit. The available studies have mostly used between 100–150 μg, with higher doses showing side effects, including sedation, bradycardia and hypotension [5,15]. Multiple studies have clearly demonstrated that the perineural effect of block prolongation is not α2-mediated. Instead, the peripheral effects of clonidine are through inhibition of the hyperpolarization-activated cation current (Ih current). This current normally functions to restore nerves from a hyperpolarized state to resting potential for a subsequent action potential. The effect appears to be more profound on C-fibers (pain fibers) than Aα fibers (motor fibers), thereby making the effects potentially more sensory specific [16–18]. Clonidine has often been used as an adjuvant to local anaesthetics in SGBs [19]. Punj [20] successfully treated a patient of systemic lupus erythematosus by administering bilateral SGBs with 5 mL of 0.25% ropivacaine and 15 μg clonidine, where a total of 64 such injections were used over a period of 35 days.
Corticosteroids have been used successfully as adjuvants to local anaesthetics in SGB in multiple studies [21,22]. Methylprednisolone acetate (depo-methylprednisolone) has been studied as an additive to local anaesthetics. Its analgesic effect is mainly mediated by acting on the locally released inflammatory mediators at the site of nerve injury. Hence, it has been studied and found to be effective in neuropathic pain states following nerve injuries [23]. Klein and Klein [24] were able to successfully treat CRPS of the upper limb by performing an ulnar nerve block within the axillary sheath, using low volume 0.5% bupivacaine with epinephrine 1:200000- 2.5 mL along with 40 mg methylprednisolone. They used 6 repeated blocks of this type over a period of 8 weeks.
Methylprednisolone was found to be better than clonidine in improving the range of motion in the present study. This may be taken into account while performing the hand exercises, which are imperative in breaking the vicious cycle of pain, immobility, decreased ROM, and pain.
In the present study, it was observed that good pain relief (greater than 50% improvement in VAS score) was experienced in both groups after completion of the series of blocks, and it persisted until 1.5 months. There was a significantly greater improvement in the ROM of the wrist joint in those who received methylprednisolone than those who received clonidine (P = 0.049) at 1.5 months after receiving the last block. However, there was no significant difference between the two groups with respect to edema score, Quick DASH score, BPI score, and Patient Satisfaction Score. Normal blood pressure, heart rate, ECG findings, and SpO2 values were noted in both the groups at all times. The skin temperature of hand was raised in all patients after each block (mean rise between 1.3 to 2°C).
Datta et al. [21] studied the effect of the SGB on upper limb CRPS. They concluded that SGBs are relatively safe and provided effective management in patients with neuropathic conditions already on pharmacotherapy. Serial blocks attained an average reduction in pain by > 3 numerical pain rating scale points from the baseline for both spontaneous and provoked pain with a decrease in mean DASH score and improvement in ROM.
Hakim et al. [25], in their study, found that there was a significant difference in the VAS score reduction between the two groups, one receiving only a steroid (dexamethasone) and the other group receiving clonidine and steroid for a lumbar sympathetic block for the treatment of lower limb CRPS type I.
In the present study, one patient in the methylprednisolone group showed a reduced heart rate from 71/min to 45/min after the fifth block, but was asymptomatic and self-limited. One subject experienced drowsiness lasting for 1–2 hours after each block that contained clonidine as an additive. There was no incidence of dysphagia, haematoma formation, difficulty in breathing, paraesthesia of the upper limb, or intrathecal injection.
In a study by Datta et al. [21], minor, self-limiting complications, such as hoarseness, dysphagia, local hematoma, and ipsilateral brachial plexus block occurred in 11.5%. A rare complication of contralateral Horner’s syndrome was documented. One patient developed a small pneumothorax, but it did not require intervention.
The authors conclude that in the present study, there was no significant difference noted between the two groups C and D, with respect to VAS scores, edema scores, Quick DASH scores, BPI scores, and overall patient satisfaction. No significant change in haemodynamic parameters nor any other complication was seen in either of the two groups during and after administration of the block. This reiterates the fact that the SGB is effective in CRPS cases where medical management fails; and ultrasonographic guidance is the safest technique for the SGB. An important finding was that the improvement in the ROM was significantly better in group D than in group C. However, the study was done at a single center with a relatively small sample size comprising of type I CRPS patients only. Hence, though it adds to the existing literature, multicentric studies with higher numbers of patients are needed to corroborate these findings. This study has some limitations. The control group that was used was not ideal, employing only local anaesthetic, without any adjuvant. However, this was done considering the established benefit with the use of adjuvants like steroids with local anaesthetics in nerve block for treating chronic pain states. Skin temperature changes following nerve block was measured using an infrared thermometer, instead of the more sensitive infrared thermography technique due to the cost involved and expertise required to interpret the results.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/EY72ZS.
REFERENCES
1. Bruehl S. 2010; An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 113:713–25. DOI: 10.1097/ALN.0b013e3181e3db38. PMID: 20693883.
2. Schwartzman RJ, McLellan TL. 1987; Reflex sympathetic dystrophy. A review. Arch Neurol. 44:555–61. DOI: 10.1001/archneur.1987.00520170081028. PMID: 3495254.
3. van Eijs F, Stanton-Hicks M, Van Zundert J, Faber CG, Lubenow TR, Mekhail N, et al. 2011; Evidence-based interventional pain medicine according to clinical diagnoses. 16. Complex regional pain syndrome. Pain Pract. 11:70–87. DOI: 10.1111/j.1533-2500.2010.00388.x. PMID: 20807353.
4. Brummett CM, Williams BA. 2011; Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 49:104–16. DOI: 10.1097/AIA.0b013e31820e4a49. PMID: 21956081. PMCID: PMC3427651.
5. Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. 2009; Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology. 111:406–15. DOI: 10.1097/ALN.0b013e3181aae897. PMID: 19602964.
6. Kamble SV, Motlekar SA, D'souza LL, Kudrigikar VN, Rao SE. 2017; Low doses of amitriptyline, pregabalin, and gabapentin are preferred for management of neuropathic pain in India: is there a need for revisiting dosing recommendations? Korean J Pain. 30:183–91. DOI: 10.3344/kjp.2017.30.3.183. PMID: 28757918. PMCID: PMC5532525.
7. Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. 1995; Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth. 20:323–8. PMID: 7577781.
8. Matsumoto S. 1991; Thermographic assessments of the sympathetic blockade by stellate ganglion block (1) comparison between C7-SGB and C6-SGB in 40 patients. Masui. 40:562–9. Japanese. PMID: 2051581.
9. Matsumoto S. 1991; Thermographic assessments of the sympathetic blockade by stellate ganglion block (2)--comparison and analysis of thermographic patterns between C7-SGB and C6-SGB in 20 healthy volunteers. Masui. 40:692–701. Japanese. PMID: 2072510.
10. Malmqvist EL, Bengtsson M, Sörensen J. 1992; Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth. 17:340–7.
11. Jadon A. 2011; Revalidation of a modified and safe approach of stellate ganglion block. Indian J Anaesth. 55:52–6. DOI: 10.4103/0019-5049.76601. PMID: 21431054. PMCID: PMC3057247. PMID: 17059b310fe9486e9401ea7c12d26e37.
12. Elias M. 2000; Cervical sympathetic and stellate ganglion blocks. Pain Physician. 3:294–304. DOI: 10.36076/ppj.2000/3/294. PMID: 16906187.
13. Evans JA. 1946; Sympathectomy for reflex sympathetic dystrophy; report of twenty-nine cases. J Am Med Assoc. 132:620–3. DOI: 10.1001/jama.1946.02870460010003. PMID: 21001604.
14. Yucel I, Demiraran Y, Ozturan K, Degirmenci E. 2009; Complex regional pain syndrome type I: efficacy of stellate ganglion blockade. J Orthop Traumatol. 10:179–83. DOI: 10.1007/s10195-009-0071-5. PMID: 19888550. PMCID: PMC2784060.
15. McCartney CJ, Duggan E, Apatu E. 2007; Should we add clonidine to local anesthetic for peripheral nerve blockade? A qualitative systematic review of the literature. Reg Anesth Pain Med. 32:330–8. DOI: 10.1097/00115550-200707000-00010. PMID: 17720118.
16. Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P. 2001; Inhibition of the I(h) current in isolated peripheral nerve: a novel mode of peripheral antinociception? Muscle Nerve. 24:254–61. DOI: 10.1002/1097-4598(200102)24:2<254::AID-MUS110>3.0.CO;2-#. PMID: 11180209.
17. Leem JW, Choi Y, Han SM, Yoon MJ, Sim JY, Leem SW. 2000; Conduction block by clonidine is not mediated by alpha2-adrenergic receptors in rat sciatic nerve fibers. Reg Anesth Pain Med. 25:620–5. DOI: 10.1053/rapm.2000.16160. PMID: 11097671.
18. Gaumann DM, Brunet PC, Jirounek P. 1994; Hyperpolarizing afterpotentials in C fibers and local anesthetic effects of clonidine and lidocaine. Pharmacology. 48:21–9. DOI: 10.1159/000139158. PMID: 8309984.
19. Nascimento MS, Klamt JG, Prado WA. 2010; Intravenous regional block is similar to sympathetic ganglion block for pain management in patients with complex regional pain syndrome type I. Braz J Med Biol Res. 43:1239–44. DOI: 10.1590/S0100-879X2010007500123. PMID: 21085893.
20. Punj J. 2019; Multiple bilateral ultrasound-guided stellate ganglion blocks to treat acute vasculitis in a recently diagnosed patient of systemic lupus erythematosus. Indian J Anaesth. 63:851–5. DOI: 10.4103/ija.IJA_331_19. PMID: 31649399. PMCID: PMC6798631. PMID: 887b4bbe6a8c43b5be17188281bc9572.
21. Datta R, Agrawal J, Sharma A, Rathore VS, Datta S. 2017; A study of the efficacy of stellate ganglion blocks in complex regional pain syndromes of the upper body. J Anaesthesiol Clin Pharmacol. 33:534–40. DOI: 10.4103/joacp.JOACP_326_16. PMID: 29416250. PMCID: PMC5791271. PMID: 534d7ed2aec34b2f94b5eb0e679d299f.
22. Makharita MY, Amr YM, El-Bayoumy Y. 2012; Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician. 15:467–74. DOI: 10.36076/ppj.2012/15/467. PMID: 23159962.
23. Eker HE, Cok OY, Aribogan A, Arslan G. 2012; Management of neuropathic pain with methylprednisolone at the site of nerve injury. Pain Med. 13:443–51. DOI: 10.1111/j.1526-4637.2011.01323.x. PMID: 22313580.
24. Klein DS, Klein PW. 1991; Low-volume ulnar nerve block within the axillary sheath for the treatment of reflex sympathetic dystrophy. Can J Anaesth. 38:764–6. DOI: 10.1007/BF03008456. PMID: 1914061.
25. Hakim KYK, Abd El Fatah AM. 2014; Clonidine in lumbar sympathetic block for lower limb complex regional pain syndrome. Ain-Shams J Anaesthesiol. 7:320–6. DOI: 10.4103/1687-7934.139557.
Fig. 2
USG image showing sonoanatomy of SGB. USG: ultrasonography, SGB: stellate ganglion block, SCM: sternocleidomastoid muscle, CA: internal carotid artery, IJV: internal jugular vein, PVF: prevertebral fascia, LC: longus coli muscle, T: transverse process of C6 vertebra.
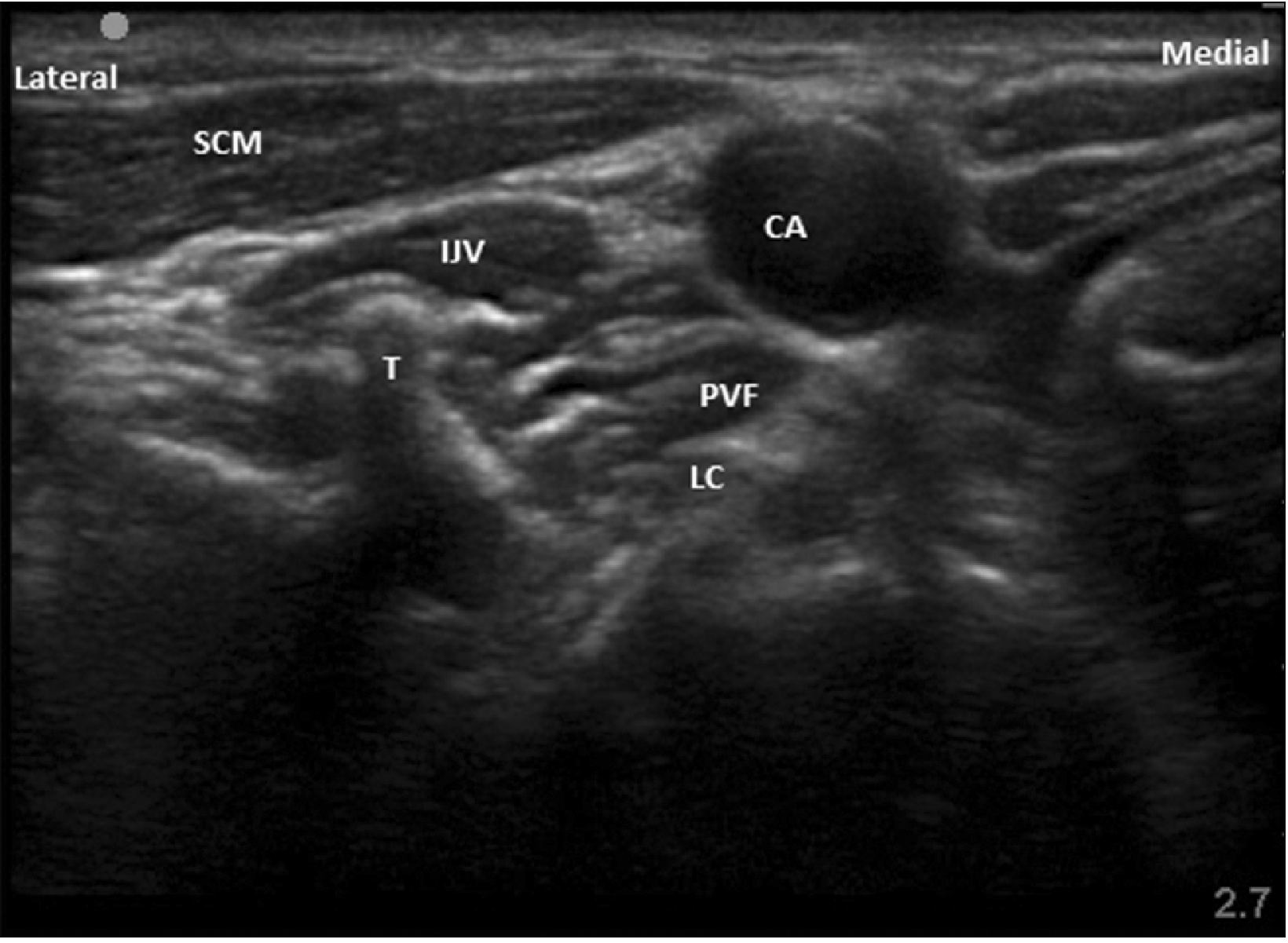
Fig. 4
Needle path in real time imaging. A: carotid artery, B: internal jugular vein, C: needle piercing the fascia covering longus colli, D: transverse process of C7, E: longus colli.
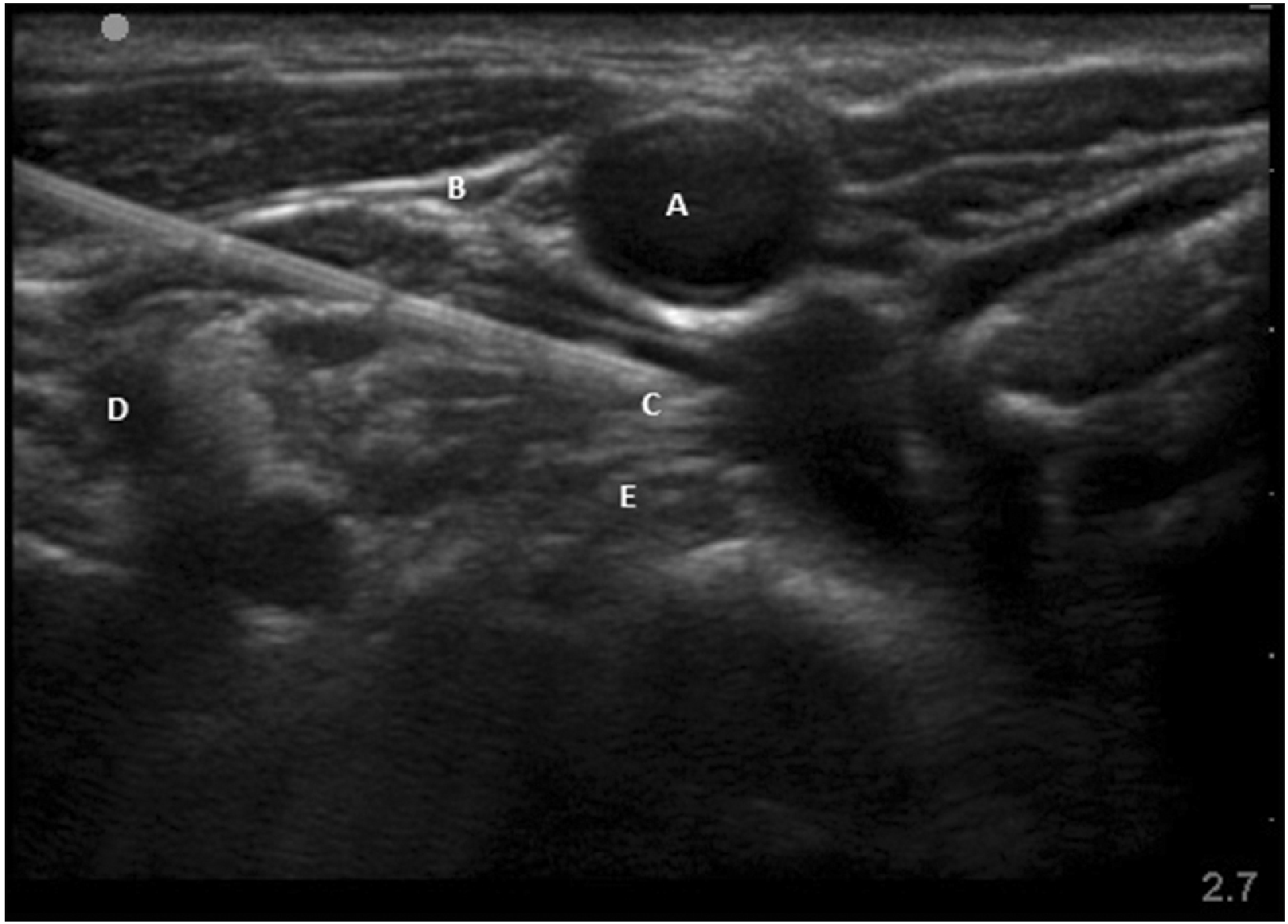
Fig. 5
Edema score at the wrist joint. Group C: subjects who were given clonidine as additive in stellate ganglion block (SGB), Group D: subjects who were given methylprednisolone as additive in SGB.
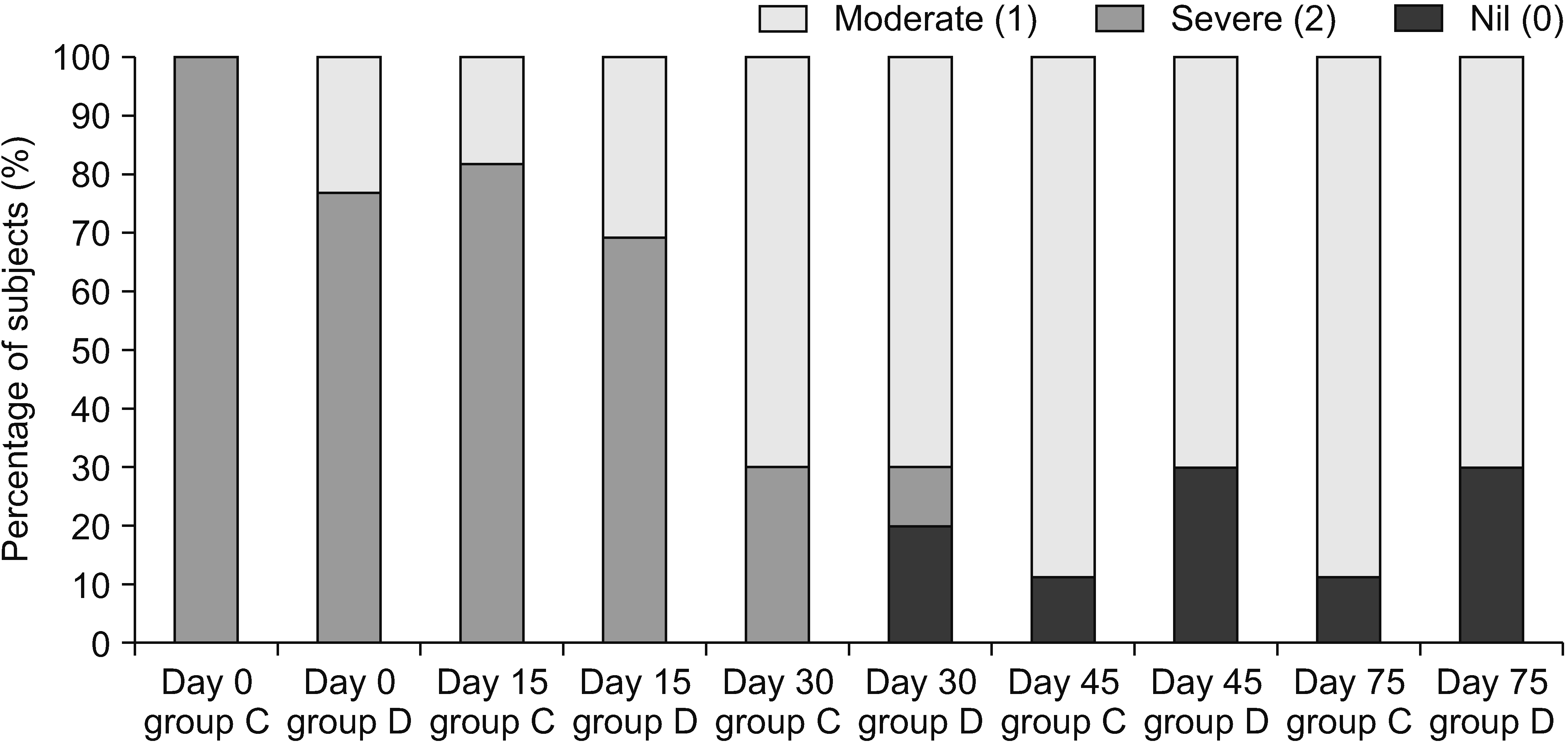
Fig. 6
Mean Quick DASH scores. DASH: Disabilities of Arm, Shoulder and Hand. Group C: subjects who were given clonidine as additive in stellate ganglion block (SGB). Group D: subjects who were given methylprednisolone as additive in SGB.
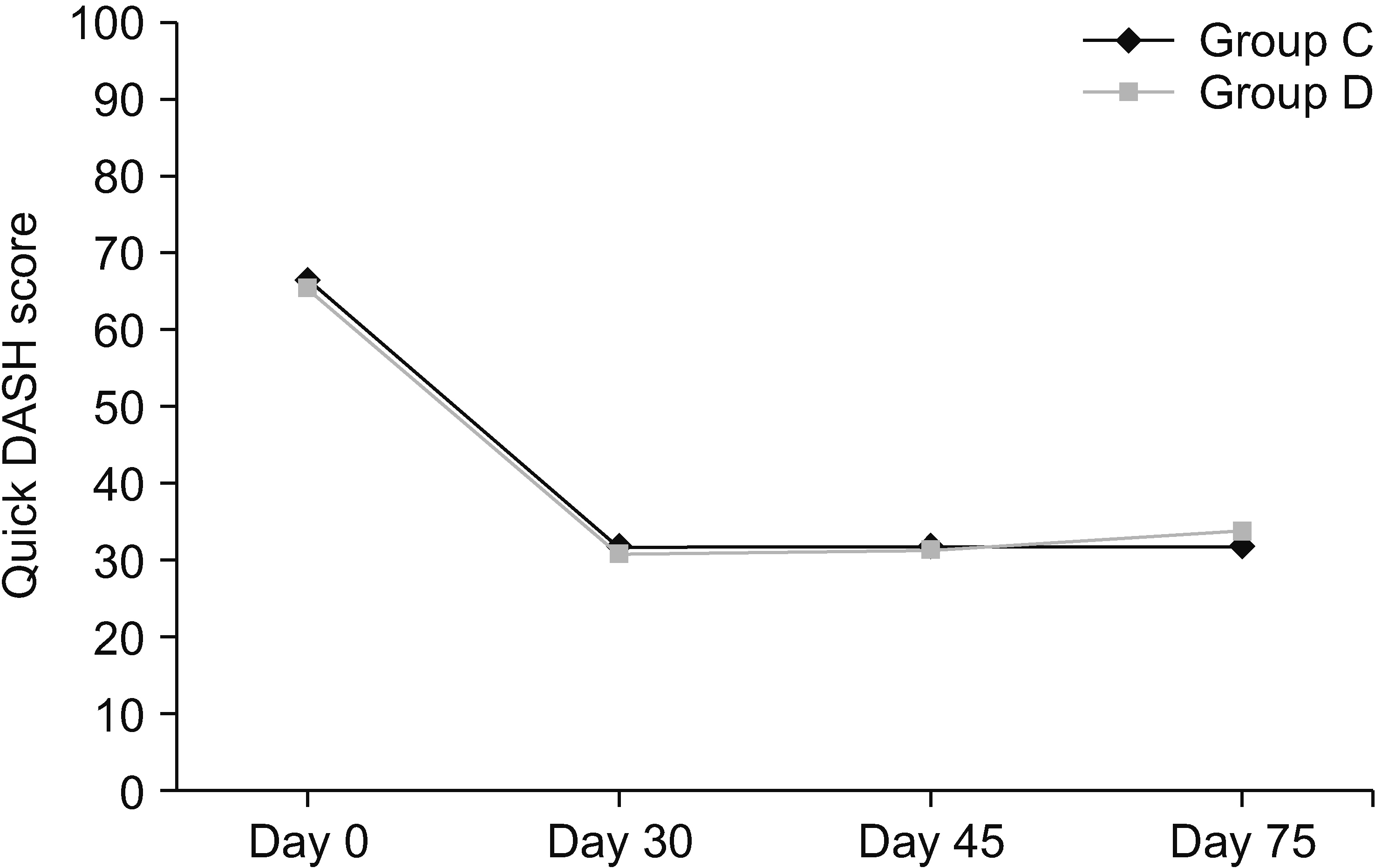
Fig. 7
Mean BPI scores. BPI: Brief Pain Inventory. Group C: subjects who were given clonidine as additive in stellate ganglion block (SGB). Group D: subjects who were given methylprednisolone as additive in SGB.
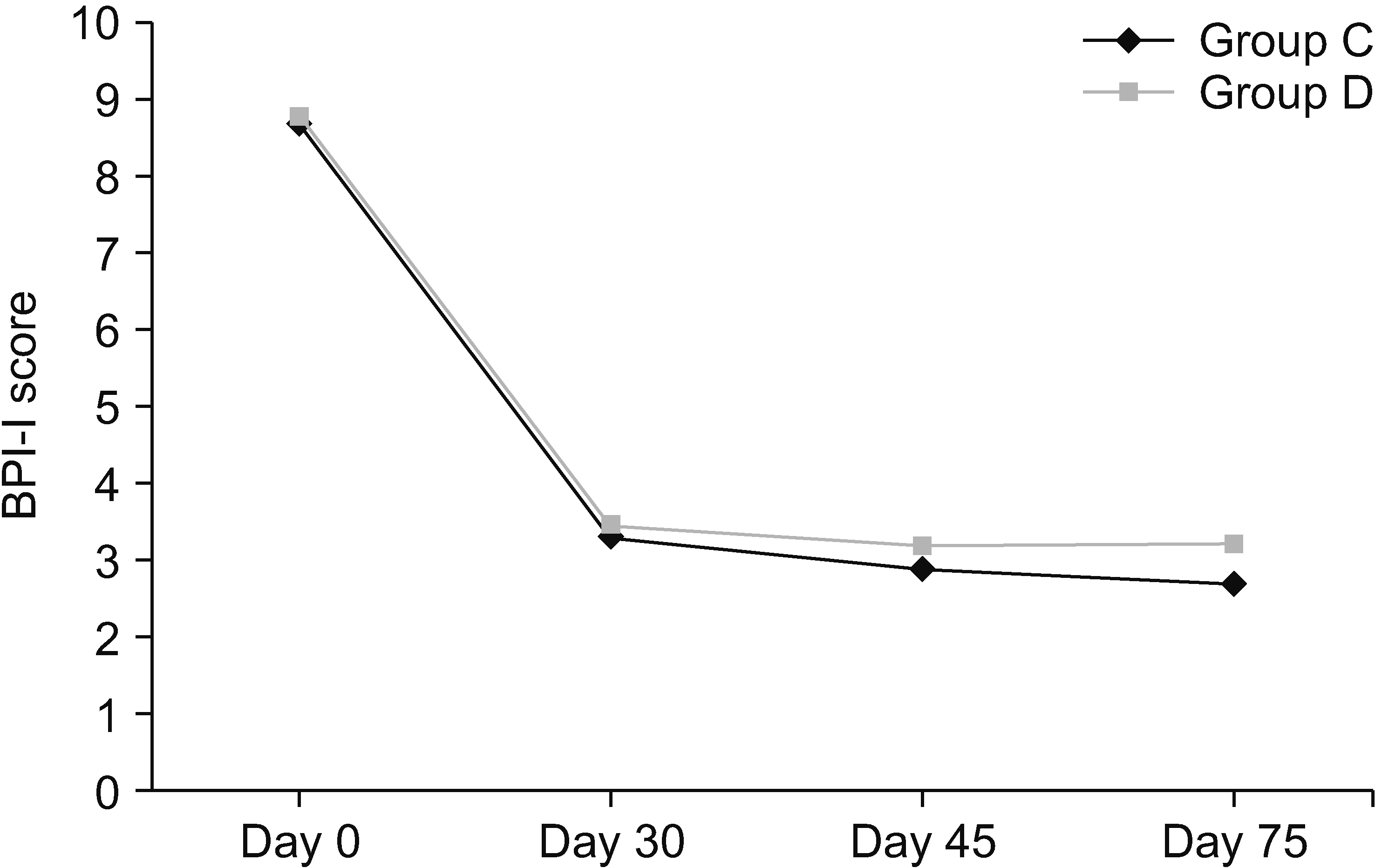
Fig. 8
Mean patient satisfaction scores. Group C: subjects who were given clonidine as additive in stellate ganglion block (SGB). Group D: subjects who were given methylprednisolone as additive in SGB.
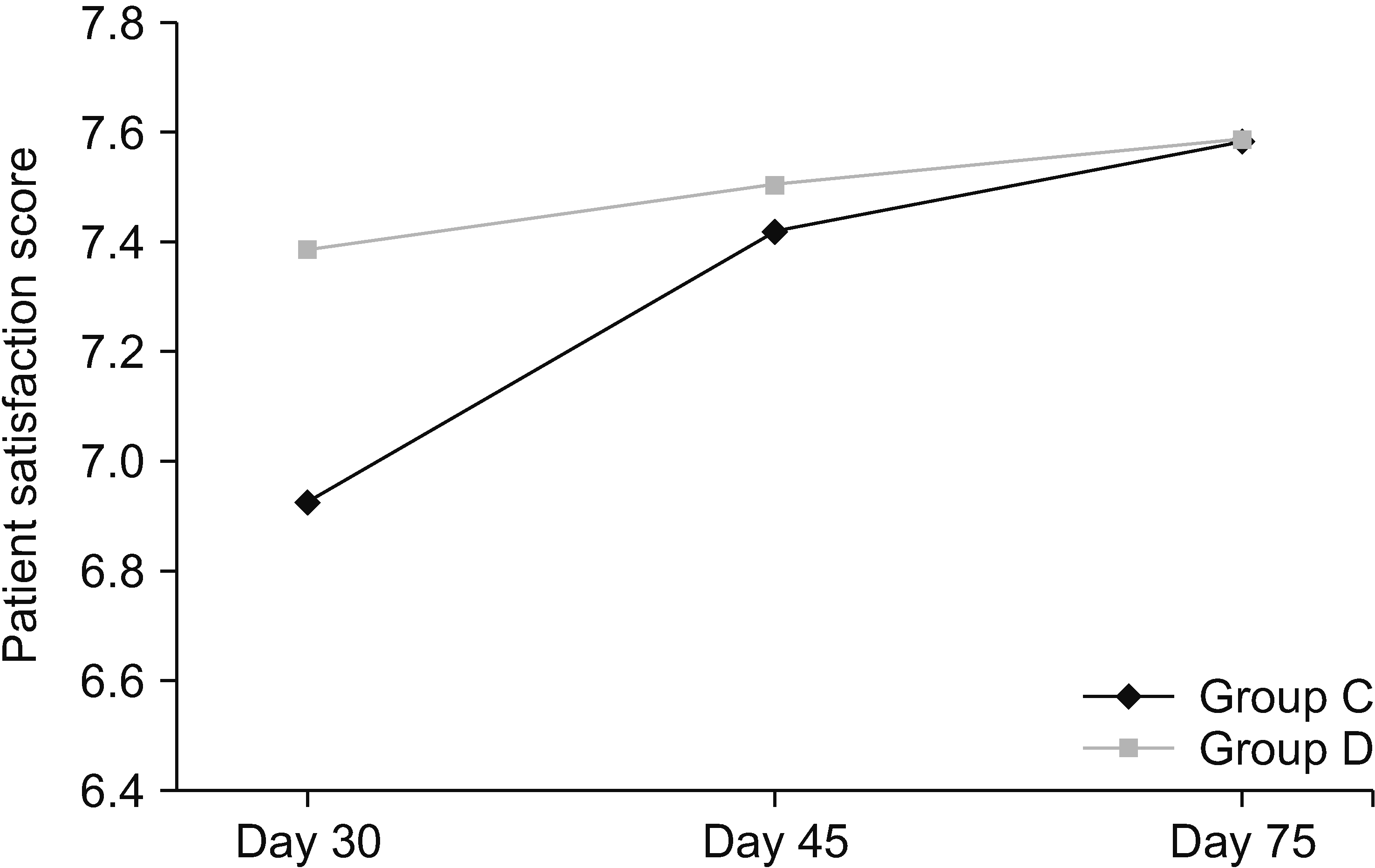
Table 1
Demographic data
| Variable | Group C (n = 14) | Group D (n = 16) | P valuea |
|---|---|---|---|
| Age (yr) | 51.7 ± 11.7 | 45.8 ± 12.8 | 0.196 |
| Sex (male/female) | 7/7 (50.0/50.0) | 7/9 (43.8/56.2) | 0.732 |
| ASA grade I/II/III | 14/0/0 | 15/1/0 | - |
| Shoulder joint involvement | 1 (7.1) | - | - |
| Elbow joint involvement | 1 (7.1) | - | - |
| Wrist joint involvement | 11 (78.6) | 13 (81.3) | - |
| Small joints involvement | 12 (85.7) | 15 (93.8) | - |
| Duration of injury (mo) | 3.6 ± 1.5 | 3.3 ± 1.5 | 0.524 |
| Mode of injury | |||
| Wrist fracture | 10 (71.4) | 10 (62.5) | |
| Dupuytrens contracture release | 1 (7.1) | 3 (18.8) | |
| Other modes of injury | 3 (21.4) | 3 (18.8) | |
| Bone scan reported | 7 (50.0) | 8 (50.0) | |
| History of analgesic intake | 1 (7.1) | 1 (6.3) | |
| Previous interventional treatment received | - | - |
Table 2
VAS score at the wrist joint
| Day | Rest/movement | Group C VAS | Group D VAS | Group C VAS | Group D VAS | P valuea |
|---|---|---|---|---|---|---|
| 0 | Rest | 8.63 ± 0.674 | 8.69 ± 0.751 | 9 (8–9) | 9 (8–9) | 0.566 |
| Movement | 9.36 ± 0.674 | 9.23 ± 0.926 | 9 (9–10) | 10 (9–10) | 0.608 | |
| 15 | Rest | 7.54 ± 1.293 | 7.46 ± 1.506 | 8 (7–8.5) | 8 (6.5–9) | 0.787 |
| Movement | 8.36 ± 1.361 | 8.307 ± 1.493 | 8 (7.5–9.5) | 8 (7.5–10) | 0.736 | |
| 30 | Rest | 3.7 ± 3.111 | 3.2 ± 1.947 | 4 (3.25–4) | 3 (3–4) | 0.257 |
| Movement | 4.2 ± 3.747 | 3.5 ± 2.368 | 4 (4–4.75) | 3.5 (3–4) | 0.120 | |
| 45 | Rest | 3.55 ± 0.881 | 3.2 ± 1.316 | 4 (3–4) | 3 (3–4) | 0.509 |
| Movement | 3.66 ± 0.866 | 3.8 ± 1.549 | 4 (3–4) | 4 (3–4) | 0.776 | |
| 75 | Rest | 3.55 ± 0.881 | 3.6 ± 1.264 | 4 (3–4) | 4 (3–4) | 0.738 |
| Movement | 3.55 ± 0.881 | 3.9 ± 1.286 | 4 (3–4) | 4 (3–4) | 0.206 |
Table 3
VAS score at the small joints
| Day | Rest/ movement | Group C VAS | Group D VAS | Group C VAS | Group D VAS | P valuea |
|---|---|---|---|---|---|---|
| 0 | Rest | 8.33 ± 0.492 | 8.53 ± 0.743 | 8 (8–9) | 9 (8–9) | 0.225 |
| Movement | 9.08 ± 0.792 | 8.93 ± 0.883 | 9 (8.75–10) | 9 (8–10) | 0.818 | |
| 15 | Rest | 7.25 ± 1.484 | 7.33 ± 1.496 | 7.5 (6–8.25) | 7 (6–9) | 0.696 |
| Movement | 7.75 ± 1.215 | 7.86 ± 1.505 | 8 (6.75–9) | 8 (7–9) | 0.576 | |
| 30 | Rest | 3.27 ± 1.190 | 3.33 ± 1.37 | 3 (2.5–4) | 3 (3–3.5) | 0.860 |
| Movement | 3.36 ± 1.501 | 3.75 ± 1.864 | 3 (2.5–4.5) | 3.5 (3–4) | 0.563 | |
| 45 | Rest | 3 ± 1.154 | 3.18 ± 1.250 | 3 (2.25–3.75) | 3 (3–4) | 0.497 |
| Movement | 3.1 ± 1.370 | 3.63 ± 1.433 | 3 (2.25–3.75) | 4 (3–4) | 0.101 | |
| 75 | Rest | 2.8 ± 1.475 | 3.63 ± 1.286 | 3 (2.25–3.75) | 4 (3–4) | 0.051 |
| Movement | 2.9 ± 1.286 | 3.63 ± 1.286 | 3 (2.25–3.75) | 4 (3–4) | 0.056 |
Table 4
Angle of wrist extension
| Day | Group C Angle (°) | Group D Angle (°) | Group C Angle (°) | Group D Angle (°) | P value |
|---|---|---|---|---|---|
| 0 | 20.83 ± 4.687 | 23.33 ± 3.892 | 20 (17.5–25) | 25 (25–25) | 0.018a |
| 15 | 21.66 ± 5.365 | 23.33 ± 3.892 | 22.5 (17.5–25) | 25 (25–25) | 0.018a |
| 30 | 47.5 ± 16.541 | 64.5 ± 13.632 | 45 (45–75) | 60 (45–60) | 0.672 |
| 45 | 50.55 ± 14.240 | 65 ± 14.142 | 45 (45–75) | 60 (45–60) | 0.853 |
| 75 | 50.55 ± 14.240 | 65 ± 14.142 | 45 (45–80) | 60 (45–60) | 0.853 |
Table 5
Angle of radial deviation
| Day | Group C Angle (°) | Group D Angle (°) | Group C Angle (°) | Group C Angle (°) | P value |
|---|---|---|---|---|---|
| 0 | 10 ± 4.264 | 12.5 ± 3.988 | 10 (10–10) | 10 (10–17.5) | 0.071 |
| 15 | 11.25 ± 5.276 | 12.91 ± 3.964 | 10 (10–10) | 10 (10–20) | 0.014a |
| 30 | 17.5 ± 4.249 | 17.5 ± 2.635 | 20 (15–20) | 17.5 (20–20) | 0.078 |
| 45 | 18.88 ± 4.166 | 17.5 ± 2.635 | 20 (15–20) | 17.5 (20–20) | 0.102 |
| 75 | 18.88 ± 4.166 | 17.77 ± 2.635 | 20 (15–20) | 20 (20–20) | 0.049a |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download