Abstract
Background
Cancer-induced bone pain (CIBP) is considered to have both nociceptive and neuropathic components. However, the prevalence, risk factors, and impact of the neuropathic components are yet poorly understood.
Methods
We estimate the prevalence of neuropathic pain (NP) features in patients with CIBP at a tertiary care pain clinic setting using the Douleur Neuropathique 4 questionnaire and evaluate their associated factors and their impact after 4 weeks of treatment using the Brief Pain Inventory questionnaire and the Edmonton Symptom Assessment System.
Results
A total of 133 patients were recruited. The estimated prevalence of NP was 30.8% (95% confidence interval 23.6%–39.1%). Initially, the patients with NP had significantly higher average pain scores (6.00 vs. 5.05, P = 0.006), higher total interference scores (5.84 vs. 4.89, P = 0.033), and symptom distress scores (35.88 vs. 26.52, P = 0.002). After 4 weeks of treatment, patients in both groups reported significantly decreased pain intensity and improved quality of life. However, the patients with NP still reported significantly higher average pain (4.61 vs. 3.58, P = 0.048), trending toward higher total interference scores (3.52 vs. 2.99, P = 0.426), and symptom distress scores (23.30 vs. 20.77, P = 0.524). From multivariate analysis, the independent risk factors for NP were younger age, pain in the extremities, and higher average pain scores.
Metastatic bone disease is a common problem in cancer patients, with high incidence in multiple myeloma, breast, prostate, thyroid, kidney, and lung cancer [1]. It often substantially affects quality of life and survival of the patients. In the majority of patients, bone metastasis is usually symptomatic, and pain is considered one of the most frequent symptoms experienced by these patients. It is estimated that severe pain, experienced by 50%–84% of patients with bone metastasis [2,3], is a common cause of hospital admissions and is associated with increased morbidity, impaired performance status, and decreased quality of life.
Even though nociceptive pain caused by mediators produced from tumor and inflammatory cells is thought to be a major component of metastatic bone pain [4], current evidence also demonstrates that neuropathic mechanisms play an important role in the pathophysiology of cancer-induced bone pain (CIBP). In animal models, it was shown that primary afferent nerve fibers innervating bone marrow and mineralized bone were injured by the invading cancer cells [5]. This was also accompanied by several neurochemical changes which had been reported following peripheral nerve injury in non-cancer subjects [6,7]. These mechanisms cause metastatic bone pain refractory to standard pain treatment.
At present, there are several studies attempting to examine the prevalence of neuropathic pain features in bone metastases [8–11]. Since they were conducted mainly in palliative radiotherapy clinics, their results cannot be generalized to other settings. To our knowledge, none of them were performed in a pain clinic setting. In addition, the results from those previous studies were still inconsistent, and only a few of them focused on the symptom burden associated with neuropathic pain features in terms of pain severity and functional interference. Moreover, none of the studies has evaluated the subsequent impact of neuropathic pain features in CIBP.
The primary objective of our study was to estimate the prevalence of neuropathic pain features in patients with CIBP in a pain clinic setting using the Douleur Neuropathique 4 (DN4) questionnaire. The secondary objectives were to evaluate their associated risk factors and to assess their effects on patient’s functions and common symptoms in cancer patients by using the Brief Pain Inventory questionnaire (BPI) and the Edmonton Symptom Assessment System (ESAS) at baseline and 4 weeks later.
Our study was a prospective cross-sectional study conducted between January 2019 and December 2020 at the pain clinic of Siriraj Hospital, a tertiary care teaching hospital in Bangkok, Thailand. The ethical approval was obtained from the Faculty of Medicine Siriraj Hospital, Mahidol University Institutional Review Board (CoA no. 545/2560 [EC4]). Written informed consent was obtained from all participants before participating in the study.
All participants were 18 years old or older. Patients referred to our pain clinic and diagnosed with CIBP were eligible. CIBP was diagnosed when the patients had pain corresponding with radiographic evidence of bone metastasis (bone scintigraphy, single-photon emission computed tomography, computerized tomography, or magnetic resonance imaging). Other potential causes of pain, such as myofascial pain, were ruled out before the diagnosis was made. The exclusion criteria were obvious neurological involvement, such as spinal cord compression, cauda equina syndrome, or peripheral nerve compression. Patients who had preexisting neurological deficits in the same area of the CIBP or cognitive dysfunction were also excluded.
Demographic data, information of relevant history, physical examination, investigation, clinical diagnosis, and cancer treatment history were obtained from electronic medical records. Any neurotoxic chemotherapy, radiotherapy, or surgery at the area of the CIBP received within a 6-month period before evaluation were noted. Data on current analgesic medications, including adjuvants, such as gabapentinoids, antidepressants, benzodiazepines, and corticosteroids were collected. Treatment with bisphosphonates within the previous 3-month period was also noted.
The skeleton was classified into 5 regions: 1) skull; 2) cervical-thoracic-lumbar spine; 3) pelvis including sacrum; 4) chest wall including rib, sternum, clavicle and scapula; and 5) upper and lower extremities. The location of the bone metastasis demonstrated by imaging study and the most severe site of the CIBP were documented.
The patients were then assessed for the pain characteristics of the most severe site and its impact. All participants were asked to complete the DN4 [12], the BPI [13], and the ESAS [14] at baseline and at 4 weeks later. The DN4 is a ten-item questionnaire used for diagnosing neuropathic pain, which is based on both the patient being interviewed about pain quality (item 1 to 7) and a physical examination performed by the physician (item 8 to 10). This questionnaire has been validated in the Thai population [15]. Patients were considered to have neuropathic pain features (neuropathic group) if the DN4 score was equal to or more than 4. Patient’s satisfaction rated on a 0–10 scale and side effects of current pain treatment were also recorded. The BPI and ESAS questionnaire were completed by patients themselves and collected by a research assistant who was blinded to the group allocation.
All patients received pain treatment from board-certified pain physicians who were blinded to group allocation, in the pain clinic of Siriraj Hospital. Treatment and medication type as well as dose depended on the physicians’ judgement, which was based on the World Health Organization Guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents [16], and a patient’s condition.
According to a previous study by Lechner et al. [10], the prevalence of neuropathic pain features in patients with bone metastasis was 25.8%. One hundred and thirty-one patients were required to provide a 95% confidence interval (CI) with a precision of 7.5%.
All statistical analyses were performed using SPSS Statistics version 18 (SPSS Inc.). Descriptive statistics are reported using mean ± standard deviation (for normally distributed data), and median and interquartile range (for non-normally distributed data). Categorical data are presented as frequency and percentage. Comparisons between the two groups (either between the neuropathic group and the non-neuropathic group or between baseline and week 4) were performed using the Student’s t-test for normally distributed continuous variables, the Mann–Whitney U-test for non-normally distributed continuous variables, and the Pearson chi-square test or Fisher exact test for categorical variables. Multivariate analysis was performed using binary logistic regression model analysis to explore the relative contributions of the various risk factors. The factors that produced a point estimate at a P value of less than 0.1 in univariate analysis were entered into multivariate regression analysis. The adjusted odds ratio (OR) with 95% CI was calculated. A P value of less than 0.05 was considered statistically significant.
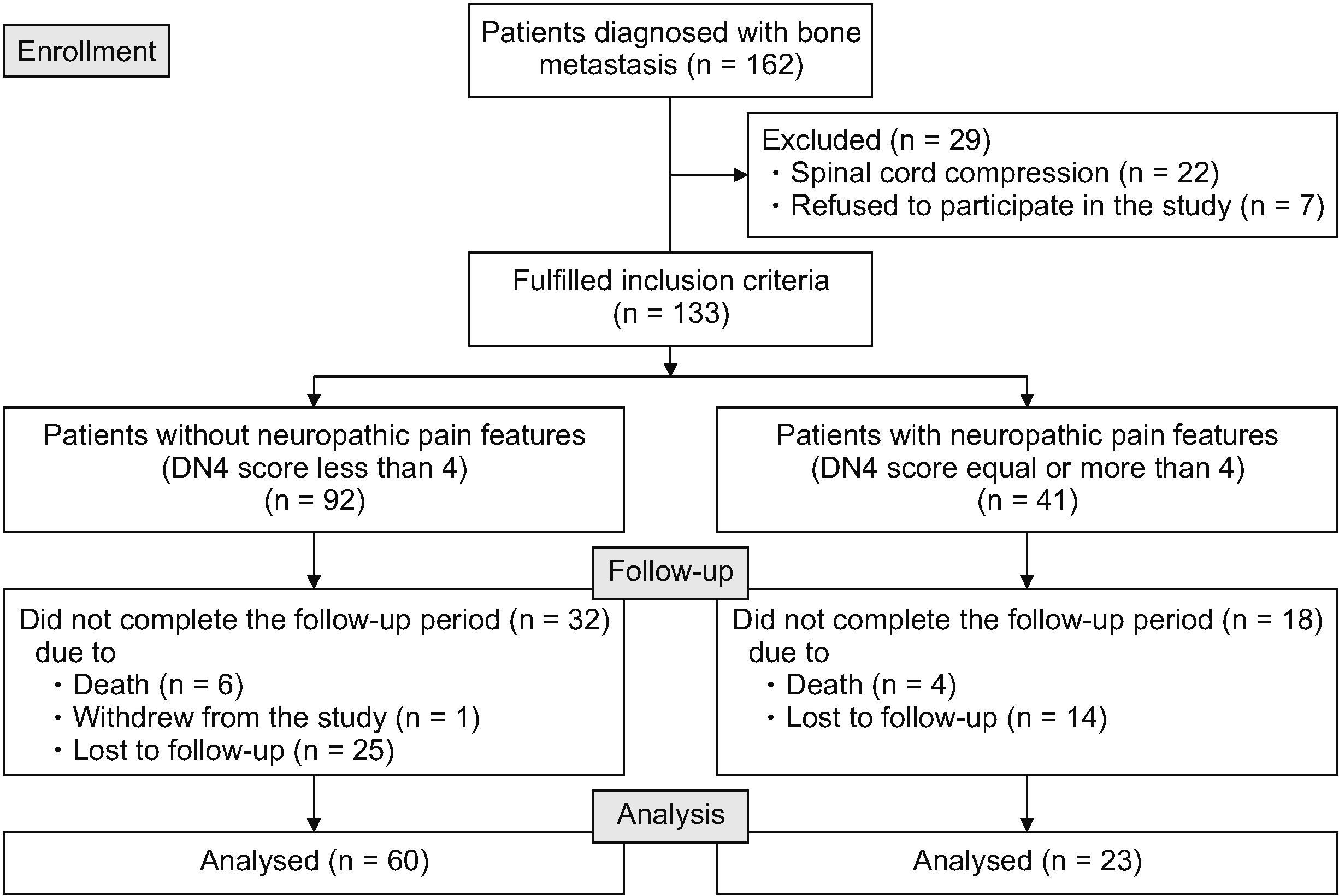
During the study period, there were 162 patients diagnosed with CIBP. Twenty-nine patients were excluded according to the exclusion criteria, and 133 patients were enrolled. A CONSORT diagram describing our study protocol is shown in Fig. 1. The demographic data and patient characteristics are presented in Table 1. The mean age of the patients was 59.1 years, and 55.6% of them were female. The most common cancers in our study were lung (26.3%) and breast cancer (24.8%). Extraosseous metastasis was found in 72.9% of patients. The majority of patients (64.7%) had at least 3 regions of bone metastasis, and the most frequently reported site of CIBP was the pelvis at 33.1%. The median of the Karnofsky Performance Status score was 70, with P25 of 60 and P75 of 80.
The majority of patients in both groups received opioid analgesics (88.0% in the non-neuropathic group and 92.7% in the neuropathic group), and morphine was the most frequently used (42.4% in the non-neuropathic group and 46.3% in the neuropathic group). The median oral morphine milligram equivalent dose was 25 mg in the non-neuropathic group and 30 mg in the neuropathic group. Gabapentinoids were prescribed 46.7% in the non-neuropathic group and 39.0% in the neuropathic group. All analgesics used by the patients in each group at the time of evaluation are summarized in Table 2. There was no statistical significance in the use of analgesic medications between the 2 groups.
The estimated prevalence of neuropathic pain features in this study was 30.8% (95% CI, 23.6% to 39.1%). The itemized response rates of DN4 are shown in Fig. 2. The most common pain characteristics in the neuropathic group were tingling (82.9%), followed by numbness (78.0%), electrical shocks (75.6%), and pins and needles (75.6%), respectively, while painful cold was the least frequent symptoms at 17.1%. Sensitivity, specificity, predictive values, and likelihood ratios of each item of the DN4 questionnaire compared with the total DN4 scores are shown in Table 3. Hypoesthesia to pinprick had the highest positive likelihood ratio.
The results of the BPI assessment are listed in Table 4. In terms of pain severity and pain-related interference, patients with neuropathic pain features reported significantly higher average pain scores than those without neuropathic pain features (6.00 vs. 5.05, respectively, P = 0.006). Additionally, BPI mean interference scores were significantly greater in the neuropathic group compared with the non-neuropathic group (5.84 vs. 4.89, respectively, P = 0.033). Scores of all 7 items of pain interference were less favorable in the neuropathic group; however, only scores of relations with other people met the statistically significant difference (P = 0.050).
Table 5 shows the results of ESAS assessment, patient’s satisfaction, and adverse effects related to current pain treatment. Patients with neuropathic pain features reported significantly higher severity of various symptoms, including tiredness, depression, anxiety, drowsiness, and shortness of breath. Total symptom distress scores in the neuropathic group were significantly higher than those in the non-neuropathic group (35.88 vs. 26.52, respectively, P = 0.002). There was no statistically significant difference in patient’s satisfaction and side effects of pain treatment between the two groups.
The univariate and multivariate analyses of risk factors for neuropathic pain features in CIBP are shown in Table 6 and Table 7, respectively. The multivariate analysis showed that risk factors found to be significantly associated with neuropathic pain features were younger age (OR, 0.962; 95% CI, 0.928 to 0.997), pain in the extremities (OR, 4.113; 95% CI, 1.221 to 13.848), and higher average pain score (OR, 1.323; 95% CI, 1.032 to 1.689). Nevertheless, daily opioid consumption showed a level of significance only in univariate analysis.
Table 8 shows pain treatment modalities which patients received during a 4-week period. There is no significant difference in the proportion of opioids, gabapentinoids, tricyclic antidepressants, prescription drugs, or other treatments between the two groups.
After the 4-week period of pain treatment, patients in both groups reported a significant decrease of pain intensity and improvement of BPI and ESAS scores (Figs. 3–5). However, patients with neuropathic pain features still reported significantly higher average pain scores (4.61 vs. 3.58, respectively, P = 0.048) and higher worst pain scores (8 vs. 5, respectively, P = 0.002) than those without neuropathic pain features. Additionally, there were trends toward higher total BPI interference scores (3.52 vs. 2.99, P = 0.426), and total ESAS symptom distress scores (23.30 vs. 20.77, P = 0.524), without statistically significant differences between the groups, as shown in Table 4 and Table 5.
In terms of changes in BPI and ESAS scores over the 4-week period, there was less improvement in the severity of tiredness and depression after pain treatment in the neuropathic group compared with the non-neuropathic group (P = 0.005 and P = 0.038, respectively). No statistically significant differences were found in the changes of other BPI and ESAS scores (Figs. 6, 7).
In the present study, about 30.8% of patients with CIBP referred to the pain clinic had neuropathic pain features. These patients had significantly higher average pain scores and greater impact on their functions at the initial visit. After 4 weeks of treatment, both groups of patients, with or without neuropathic features, reported decreased pain intensity and improved quality of life. However, pain intensity was still higher in patients with neuropathic pain features.
CIBP has distinctive and complex mechanisms. Current evidence has demonstrated the neuropathic mechanisms involved in this type of pain [17]. These mechanisms include cancer-induced damage to sensory fibers, pathological remodeling of the peripheral nervous system, and the consequences of cancer treatments, such as chemotherapy, radiotherapy, or surgery [17–20]. Moreover, both peripheral and central sensitization caused by afferent pain impulses can result in hyperexcitability of the dorsal horn neurons in the spinal cord [19,20].
The prevalence of neuropathic pain features in cancer patients with bone metastases has been reported previously by several cross-sectional studies conducted in radiotherapy clinics. Two studies using a self-report version of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale (S-LANSS) found that the prevalence of neuropathic pain features in patients with symptomatic bone metastases were 17% and 25.8%, respectively [9,10]. Nakamura et al. [11] reported a prevalence of 24% in a cohort survey using a seven-item questionnaire developed for identifying neuropathic pain components in Japanese patients. The 30.8% prevalence in our study was slightly higher than the figures reported in other studies, but is plausible, due to the factors explained below.
Firstly, this study was actually done in different study populations. Patients were referred to our pain clinic when their pain was intractable or unmanageable. In contrast with a radiotherapy clinic setting, Lechner et al. [10] showed that 8.1% of their sample population did not receive any analgesic drugs, which suggested that pain might have been less severe in these groups of patients. Additionally, ethnicity may also have an important role in manifestation of pain and its prevalence [21,22].
Another study conducted by Habberstad et al. [8] reported a 24.4% prevalence of neuropathic pain in patients with bone metastases in a palliative care setting. Nevertheless, the neuropathic pain condition in this study was diagnosed according to the Edmonton Classifications System for Cancer, which is based on clinical judgement rather than standardized tools for neuropathic pain assessment [23]. Moreover, they did not exclude neuropathic pain from spinal cord or peripheral nerve compression. These factors may have contributed to less accurate results from the study.
A second factor affecting the prevalence of neuropathic pain features is the diagnostic tool for neuropathic features. Several tools have been developed for neuropathic pain screening, for example, the DN4, the S-LANSS, the painDETECT questionnaire, and the Neuropathic Pain Questionnaire (NPQ) [24]. However, none of them have been specifically designed for cancer patients. In the present study, instead of the S-LANSS used by previous studies, we chose the DN4 as a neuropathic pain assessment tool for several reasons. First, it consists of both subjective parameters obtained from interviewed patients and objective parameters obtained from clinical examination, which is different from the S-LANSS, which relies solely on information reported by the patient. In addition, this tool has been validated in the Thai population [15]. Finally, a systematic review also showed that the DN4 had a high accuracy in the cancer population (sensitivity 82%–87%, specificity 88%) [25]. These factors may contribute to a slightly higher prevalence than the other studies.
An additional different finding, compared to other studies, is the common manifestation of neuropathic pain. Our study showed tingling, numbness, pins and needles, and electrical shocks were the common neuropathic pain characteristics, whereas the other studies described the common manifestation as discomfort after pressing the painful area [9,10], which may result from the use of different neuropathic pain screening tools. Moreover, the authors’ findings showed that hypoesthesia to pinprick had the highest positive likelihood ratio for neuropathic pain features, which can be a clinical clue to the clinician to suspect and evaluate for a neuropathic component in a patient with CIBP.
In addition to the common and highly likely symptoms and signs, risk factors associated with neuropathic CIBP can be clues for a clinician of the possibility of neuropathic CIBP. The multivariate analysis showed that risk factors that were significantly associated with neuropathic pain features were younger age, higher average pain score, and pain in extremities. There was no significant association between neuropathic pain features and the type or duration of the cancer, the number of bone metastases, the presence of pathological fractures, or the current pain medications, which is consistent with other previous studies [9–11].
The finding from the present study, that younger patients had higher prevalence of neuropathic features, is somewhat different from neuropathic pain in some conditions, such as postherpetic neuralgia and diabetic peripheral neuropathy [26–28]. We believe that this might relate to the sensory innervation of human bone. Some immunohistochemical studies have shown that the number of sensory and sympathetic nerve fibers significantly declined with age [29,30]. As a result, the risk of sensory fiber damage might increase for young people. In addition, since pain sensitivity also diminishes with age [31,32], older people might be less likely to experience neuropathic symptoms.
Sensitization might be a possible reason why patients with higher average pain scores experienced a higher chance of neuropathic features. It has been shown that an increase in pain intensity can affect the extent of both peripheral and central sensitization [33,34], which are important neuropathic mechanisms involved in bone metastasis [19,20,35]. While a possible explanation of the association between CIBP in the extremities and neuropathic pain characteristics is still unclear, the authors believe that it might relate to a close proximity of the nerves to the long bones in extremities. Nevertheless, further study is needed to confirm this hypothesis.
A significant finding of the present study was the association of neuropathic pain features with higher pain intensity, especially average pain scores, in which there were statistically significant differences between the two groups. Also, the BPI mean interference scores were significantly greater in the neuropathic group. These results were consistent with those of previous studies. Habberstad et al. [8] reported that neuropathic pain was associated with higher both average and worst pain scores. Kerba et al. [9] and Nakamura et al. [11] demonstrated higher worst pain scores in patients with neuropathic pain features. Additionally, Kerba et al. [9] also showed higher BPI mean interference scores in the neuropathic group, but the difference was not statistically significant. These findings suggest that the pain of this group of patients is more severe, and has greater impact on patient’s functions.
More importantly, to the best of the authors’ knowledge, this study is the first prospective study to investigate the effects of neuropathic features in CIBP after the treatment. Even though, after 4 weeks of treatment, both group of patients reported significant decreases of pain intensity and improvement of pain-related interference and symptoms, pain intensity, especially average pain and worst pain, was still significantly higher in patients with neuropathic pain features. Additionally, the total pain interference scale and most of the subscale from BPI, the total symptoms distress score and most of the subscale from ESAS were higher in neuropathic pain features, but did not reach statistical significance, possibly due to the small sample size at the 4-week follow up. All of these findings highlight the fact that neuropathic CIBP is a common condition and associated with worsening patient outcomes at the time of presentation and subsequently.
There are several limitations of our study. First, even though the DN4 was demonstrated to have good accuracy in the cancer population, there may be differences in the neuropathic pain symptom profile from CIBP compared with other neuropathic cancer pain syndromes. Furthermore, according to the proposed definition of neuropathic pain by the International Association for the Study of Pain, the diagnosis of neuropathic pain should be based on the neuropathic pain grading system [36,37]. The definite diagnosis of neuropathic pain should be comprised of three criteria: 1) a history of relevant neurological lesion or disease, 2) abnormal sensory signs in the same neuroanatomically plausible distribution, and 3) a diagnostic test to confirm the lesion or disease of the somatosensory nervous system. This may not be practical in this setting, as no more than the first and the second criteria could be possibly met, and only the diagnosis of probable neuropathic pain could be made at best. Moreover, a recent study demonstrated only moderate concordance between the DN4 and the grading system in cancer patients (Cohen’s kappa concordance coefficient 0.57) [38]. However, in the cancer population, some experts pay less importance on the diagnostic confirming tests, and they suggest that the diagnosis of neuropathic pain can be made without these tests [39].
Another limitation is that our study was an observational study. Therefore, it is not possible to confirm the cause and effect between our identified risk factors and neuropathic pain features. In addition, that a significant proportion of patients, especially in the neuropathic group, were lost to follow-up might affect the validity of our study. We believe that this might be because the conditions of patients with CIBP can deteriorate at any time. Thus, further longitudinal studies are needed to confirm our results.
Also, we did not control the pain treatment including the pain medications that patients received. In our institution, anti-neuropathic medications, such as gabapentinoids or tricyclic antidepressants, were prescribed to patients with neuropathic pain features unless contraindicated. However, since several studies have demonstrated the opioid-sparing effect and the ability to reduce central sensitization of these adjuvants [40–42], these drugs were also prescribed for moderate to severe CIBP even without neuropathic features. For this reason, although patients with neuropathic features tended to receive anti-neuropathic medications more frequently, the differences were not statistically significant. This might affect the changes in characteristic of pain, as well as BPI and ESAS scores at the follow-up.
The present study emphasizes that neuropathic pain features in bone metastasis are not uncommon, especially in the pain clinic setting. They negatively affect not only pain severity, but also patient functionality. Hence, physicians should always look for and pay attention to these conditions. More importantly, further research on treatment strategies in this population needs to be undertaken.
ACKNOWLEDGMENTS
The authors thank Dr. Arunee Saengsanon for assisting with the data collection, and Ms. Nattaya Bunwatsana for assisting with the IRB processing.
Notes
REFERENCES
1. Coleman RE. 2006; Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 12(20 Pt 2):6243s–9s. DOI: 10.1158/1078-0432.CCR-06-0931. PMID: 17062708.
2. Coleman RE. 1997; Skeletal complications of malignancy. Cancer. 80(8 Suppl):1588–94. DOI: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. PMID: 9362426.
3. Hird A, Zhang L, Holt T, Fairchild A, DeAngelis C, Loblaw A, et al. 2009; Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol). 21:329–35. DOI: 10.1016/j.clon.2008.12.010. PMID: 19232483.
4. Aielli F, Ponzetti M, Rucci N. 2019; Bone metastasis pain, from the bench to the bedside. Int J Mol Sci. 20:280. DOI: 10.3390/ijms20020280. PMID: 30641973. PMCID: PMC6359191. PMID: 90356177399d4f64893f69771292eb06.
5. Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, et al. 2005; Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 193:85–100. DOI: 10.1016/j.expneurol.2004.11.028. PMID: 15817267.
6. Mantyh PW. 2006; Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 7:797–809. DOI: 10.1038/nrn1914. PMID: 16988655.
7. Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, et al. 2003; Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 101:65–77. DOI: 10.1016/S0304-3959(02)00296-8. PMID: 12507701.
8. Habberstad R, Hjermstad MJ, Brunelli C, Kaasa S, Bennett MI, Pardon K, et al. 2019; Which factors can aid clinicians to identify a risk of pain during the following month in patients with bone metastases? A longitudinal analyses. Support Care Cancer. 27:1335–43. DOI: 10.1007/s00520-018-4405-9. PMID: 30105665.
9. Kerba M, Wu JS, Duan Q, Hagen NA, Bennett MI. 2010; Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol. 28:4892–7. DOI: 10.1200/JCO.2010.28.6559. PMID: 20921451.
10. Lechner B, Chow S, Chow R, Zhang L, Tsao M, Danjoux C, et al. 2016; The incidence of neuropathic pain in bone metastases patients referred for palliative radiotherapy. Radiother Oncol. 118:557–61. DOI: 10.1016/j.radonc.2016.02.018. PMID: 26924340.
11. Nakamura N, Takahashi O, Zenda S, Kawamori J, Ogita M, Onozawa M, et al. 2016; Neuropathic pain features in patients with bone metastases. Clin Oncol (R Coll Radiol). 28:204–8. DOI: 10.1016/j.clon.2015.10.007. PMID: 26537667.
12. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. 2005; Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 114:29–36. DOI: 10.1016/j.pain.2004.12.010. PMID: 15733628.
13. Chaudakshetrin P. 2009; Validation of the Thai Version of Brief Pain Inventory (BPI-T) in cancer patients. J Med Assoc Thai. 92:34–40. PMID: 19260241.
14. Chinda M, Jaturapatporn D, Kirshen AJ, Udomsubpayakul U. 2011; Reliability and validity of a Thai version of the edmonton symptom assessment scale (ESAS-Thai). J Pain Symptom Manage. 42:954–60. DOI: 10.1016/j.jpainsymman.2011.02.020. PMID: 21621964.
15. Chaudakshetrin P, Prateepavanich P, Chira-Adisai W, Tassanawipas W, Leechavengvongs S, Kitisomprayoonkul W. 2007; Cross-cultural adaptation to the Thai language of the neuropathic pain diagnostic questionnaire (DN4). J Med Assoc Thai. 90:1860–5.
16. World Health Organization. 2018. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. World Health Organization;p. 21–49.
17. Falk S, Dickenson AH. 2014; Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol. 32:1647–54. DOI: 10.1200/JCO.2013.51.7219. PMID: 24799469.
18. Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. 2010; Bone cancer pain. Ann N Y Acad Sci. 1198:173–81. DOI: 10.1111/j.1749-6632.2009.05429.x. PMID: 20536932. PMCID: PMC5642911.
19. Mantyh PW. 2014; Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care. 8:83–90. DOI: 10.1097/SPC.0000000000000048. PMID: 24792411. PMCID: PMC4068714.
20. Zhu XC, Zhang JL, Ge CT, Yu YY, Wang P, Yuan TF, et al. 2015; Advances in cancer pain from bone metastasis. Drug Des Devel Ther. 9:4239–45. DOI: 10.2147/DDDT.S87568. PMID: 26316696. PMCID: PMC4547660.
21. Yang G, Luo Y, Baad-Hansen L, Wang K, Arendt-Nielsen L, Xie QF, et al. 2013; Ethnic differences in oro-facial somatosensory profiles-quantitative sensory testing in Chinese and Danes. J Oral Rehabil. 40:844–53. DOI: 10.1111/joor.12091. PMID: 23980926.
22. Zajacova A, Grol-Prokopczyk H, Fillingim R. 2022; Beyond Black vs White: racial/ethnic disparities in chronic pain including Hispanic, Asian, Native American, and multiracial US adults. Pain. 163:1688–99. DOI: 10.1097/j.pain.0000000000002574. PMID: 35250011.
23. Nekolaichuk CL, Fainsinger RL, Lawlor PG. 2005; A validation study of a pain classification system for advanced cancer patients using content experts: the Edmonton Classification System for Cancer Pain. Palliat Med. 19:466–76. DOI: 10.1191/0269216305pm1055oa. PMID: 16218159.
24. Mulvey MR, Bennett MI, Liwowsky I, Freynhagen R. 2014; The role of screening tools in diagnosing neuropathic pain. Pain Manag. 4:233–43. DOI: 10.2217/pmt.14.8. PMID: 24953075.
25. Mulvey MR, Boland EG, Bouhassira D, Freynhagen R, Hardy J, Hjermstad MJ, et al. 2017; Neuropathic pain in cancer: systematic review, performance of screening tools and analysis of symptom profiles. Br J Anaesth. 119:765–74. DOI: 10.1093/bja/aex175. PMID: 29121284.
26. Bouhassira D. 2019; Neuropathic pain: definition, assessment and epidemiology. Rev Neurol (Paris). 175:16–25. DOI: 10.1016/j.neurol.2018.09.016. PMID: 30385075.
27. Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al. 2016; A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 157:30–54. DOI: 10.1097/j.pain.0000000000000307. PMID: 26218719. PMCID: PMC4685754.
28. Hébert HL, Veluchamy A, Torrance N, Smith BH. 2017; Risk factors for neuropathic pain in diabetes mellitus. Pain. 158:560–8. DOI: 10.1097/j.pain.0000000000000785. PMID: 27941499. PMCID: PMC5359789.
29. Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. 2018; The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 387:178–90. DOI: 10.1016/j.neuroscience.2018.01.047. PMID: 29432884. PMCID: PMC6086773.
30. Steverink JG, Oostinga D, van Tol FR, van Rijen MHP, Mackaaij C, Verlinde-Schellekens SAMW, et al. 2021; Sensory innervation of human bone: an immunohistochemical study to further understand bone pain. J Pain. 22:1385–95. DOI: 10.1016/j.jpain.2021.04.006. PMID: 33964414.
31. González-Roldán AM, Terrasa JL, Sitges C, van der Meulen M, Anton F, Montoya P. 2020; Age-related changes in pain perception are associated with altered functional connectivity during resting state. Front Aging Neurosci. 12:116. DOI: 10.3389/fnagi.2020.00116. PMID: 32457594. PMCID: PMC7221150. PMID: d7a3dde2b1984dbc80faa896217f81a8.
32. Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. 2017; Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 75:104–13. DOI: 10.1016/j.neubiorev.2017.01.039. PMID: 28159611.
33. Richebé P, Capdevila X, Rivat C. 2018; Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 129:590–607. DOI: 10.1097/ALN.0000000000002238. PMID: 29738328.
34. Thapa P, Euasobhon P. 2018; Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 31:155–73. DOI: 10.3344/kjp.2018.31.3.155. PMID: 30013730. PMCID: PMC6037807.
35. Oostinga D, Steverink JG, van Wijck AJM, Verlaan JJ. 2020; An understanding of bone pain: a narrative review. Bone. 134:115272. DOI: 10.1016/j.bone.2020.115272. PMID: 32062002.
36. Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, et al. 2016; Neuropathic pain: an updated grading system for research and clinical practice. Pain. 157:1599–606. DOI: 10.1097/j.pain.0000000000000492. PMID: 27115670. PMCID: PMC4949003.
37. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). 2019; The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 160:53–9. DOI: 10.1097/j.pain.0000000000001365. PMID: 30586071. PMCID: PMC6310153.
38. Shkodra M, Brunelli C, Zecca E, Formaglio F, Bracchi P, Lo Dico S, et al. 2021; Neuropathic pain: clinical classification and assessment in patients with pain due to cancer. Pain. 162:866–74. DOI: 10.1097/j.pain.0000000000002076. PMID: 32947548. PMCID: PMC7920493.
39. Brunelli C, Bennett MI, Kaasa S, Fainsinger R, Sjgren P, Mercadante S, et al. European Association for Palliative Care (EAPC) Research Network and International Association for the Study of Pain (IASP) Cancer Pain Special Interest Group. 2014; Classification of neuropathic pain in cancer patients: a Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain. 155:2707–13. DOI: 10.1016/j.pain.2014.09.038. PMID: 25284070.
40. Bennett MI. 2011; Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med. 25:553–9. DOI: 10.1177/0269216310378546. PMID: 20671006.
41. Nishihara M, Arai YC, Yamamoto Y, Nishida K, Arakawa M, Ushida T, et al. 2013; Combinations of low-dose antidepressants and low-dose pregabalin as useful adjuvants to opioids for intractable, painful bone metastases. Pain Physician. 16:E547–52. DOI: 10.36076/ppj.2013/16/E547. PMID: 24077205.
42. Tuchman M, Barrett JA, Donevan S, Hedberg TG, Taylor CP. 2010; Central sensitization and Ca(V)α₂δ ligands in chronic pain syndromes: pathologic processes and pharmacologic effect. J Pain. 11:1241–9. DOI: 10.1016/j.jpain.2010.02.024. PMID: 20472509.
Fig. 2
Bar chart illustrating comparisons of the percentage of patients having each neuropathic symptom between non-neuropathic and neuropathic group. A P value of less than 0.05 indicates statistical significance.
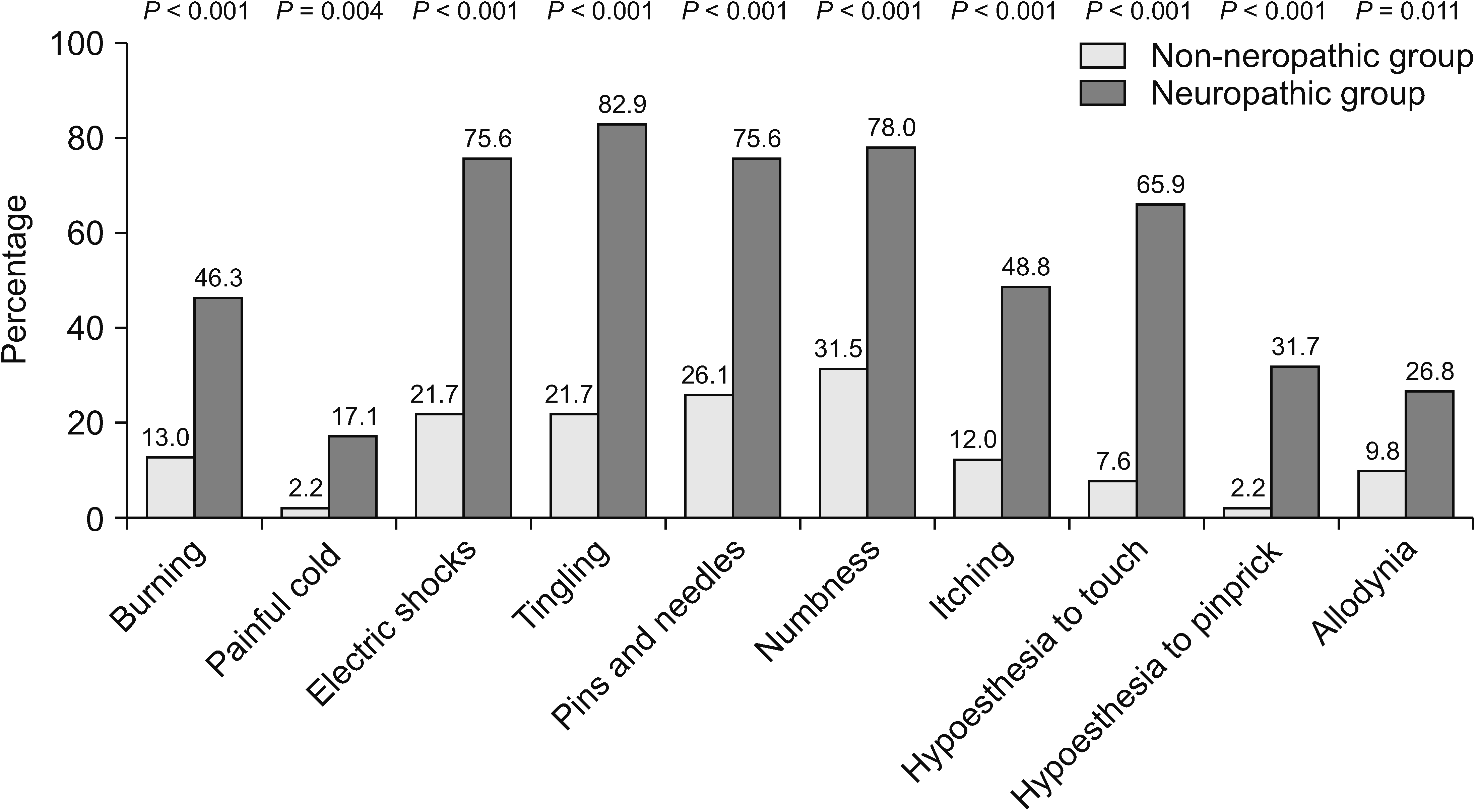
Fig. 3
Line graph showing changes of average pain score between baseline and week 4. *Independent t-test was used and a P value of less than 0.05 indicates statistically significant difference between two groups at baseline and week 4. aPaired t-test demonstrated statistically significant difference between average pain score of patients in non-neuropathic group at baseline and week 4 (P <0.001). bPaired t-test demonstrated statistically significant difference between average pain score of patients in neuropathic group at baseline and week 4 (P = 0.003).
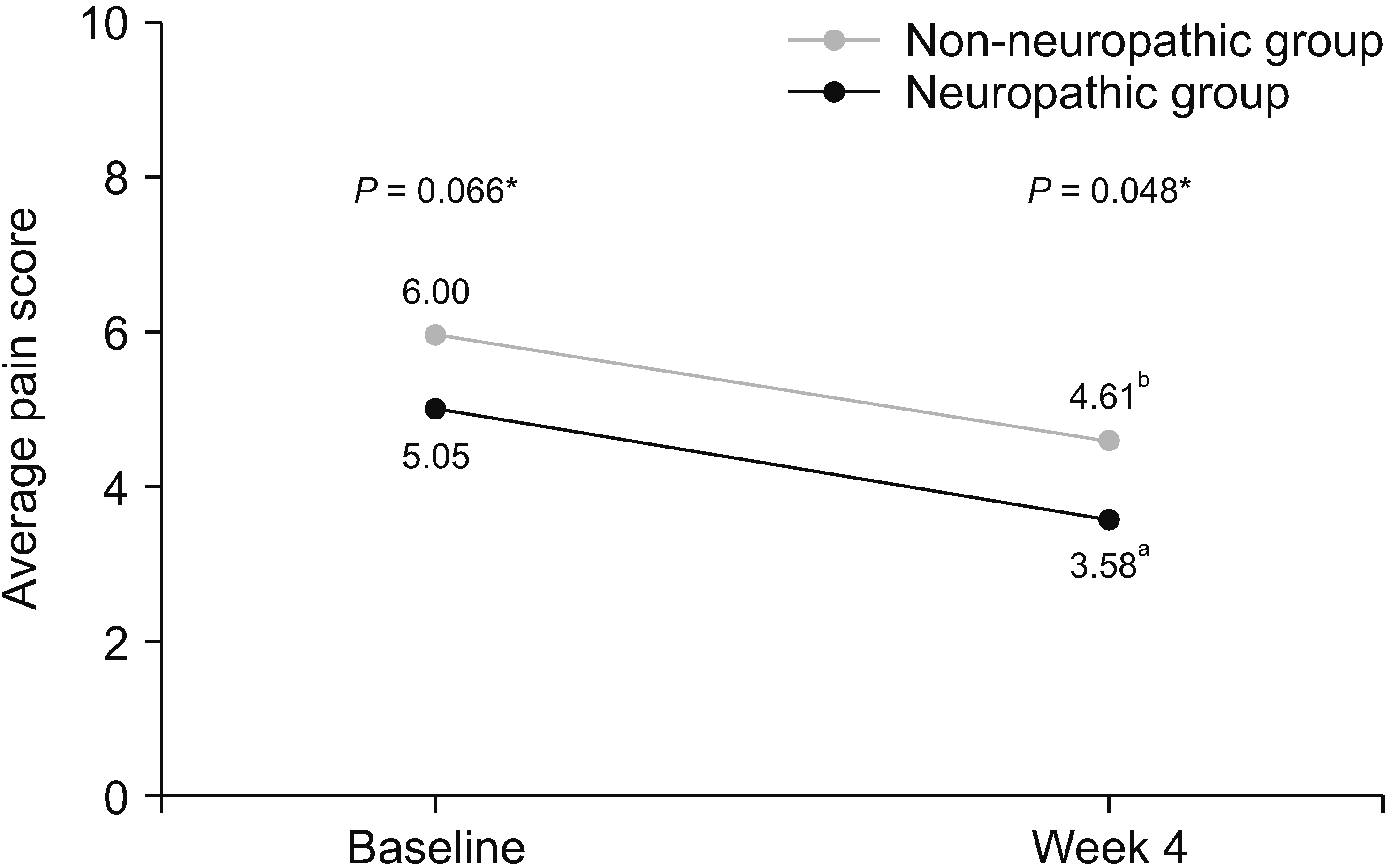
Fig. 4
Line graph showing changes of total interference score of Brief Pain Inventory between baseline and week 4. *Independent t-test was used and a P value of less than 0.05 indicates statistically significant difference between two groups at baseline. aPaired t-test demonstrated statistically significant difference between total interference score of patients in non-neuropathic group at baseline and week 4 (P <0.001). bPaired t-test demonstrated statistically significant difference between total interference score of patients in neuropathic group at baseline and week 4 (P = 0.013).
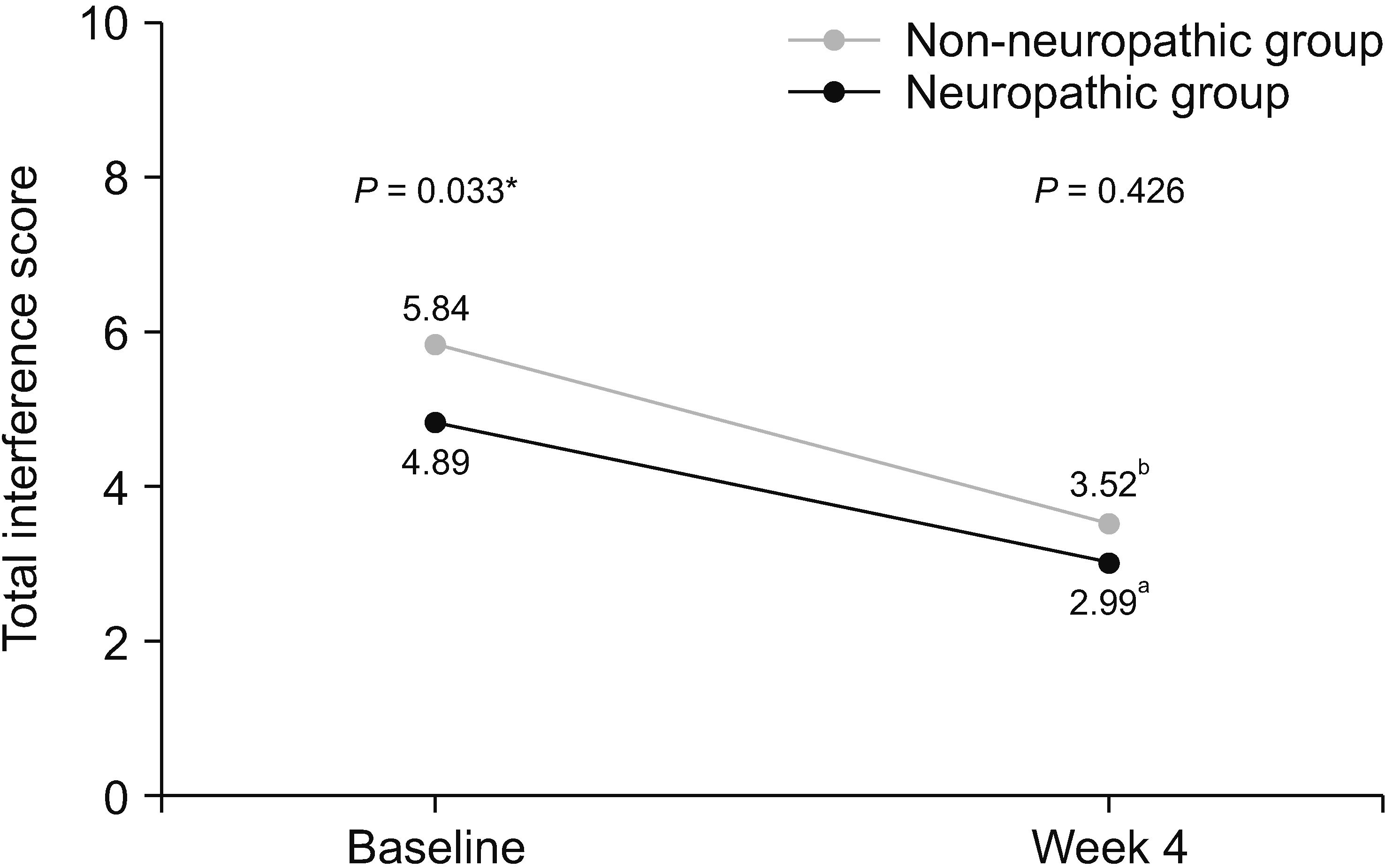
Fig. 5
Line graph showing changes of symptom distress score of Edmonton Symptom Assessment Scale between baseline and week 4. *Independent t-test was used and a P value of less than 0.05 indicates statistically significant difference between two groups at baseline. aPaired t-test demonstrated statistically significant difference between symptom distress score of patients in non-neuropathic group at baseline and week 4 (P = 0.040). bPaired t-test demonstrated statistically significant difference between symptom distress score of patients in neuropathic group at baseline and week 4 (P = 0.002).
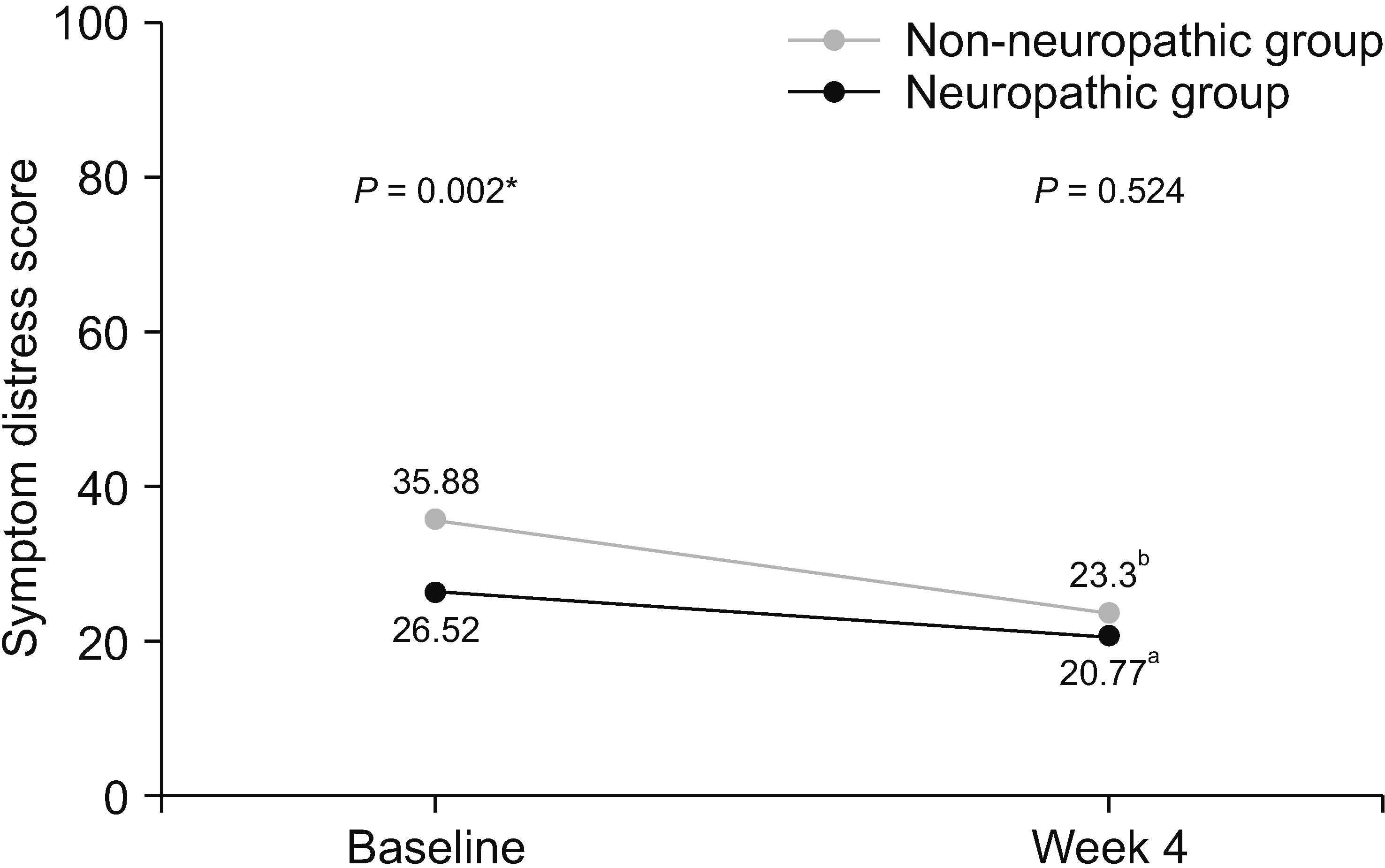
Fig. 6
Box plot showing differences of Brief Pain Inventory (BPI) between baseline and week 4. Mann–Whitney U-test was used and a P value of less than 0.05 indicates statistical significance.
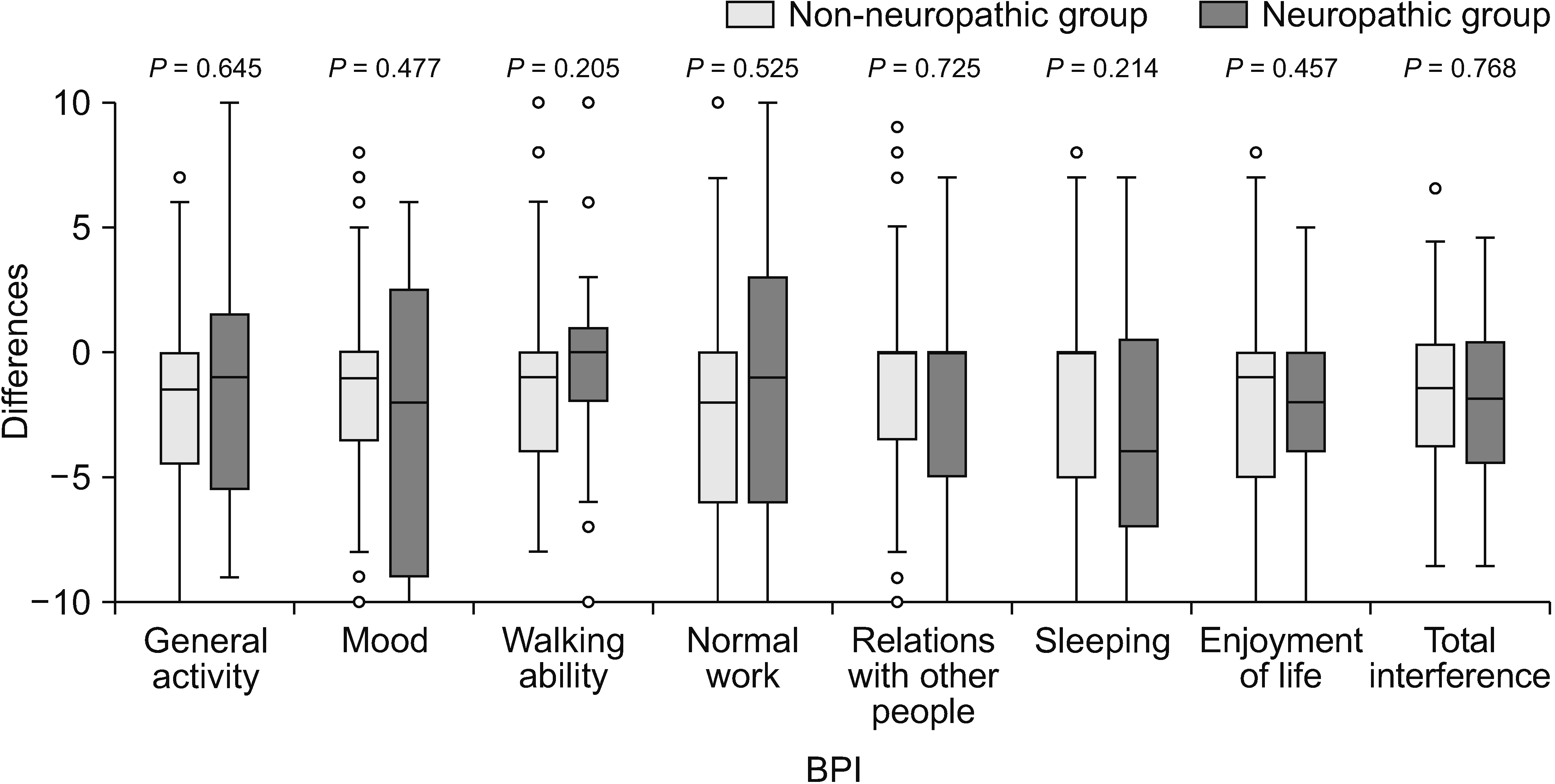
Fig. 7
Box plot showing differences of Edmonton Symptom Assessment Scale (ESAS) between baseline and week 4. Mann–Whitney U- test was used and a P value of less than 0.05 indicates statistical significance. a demonstrates statistically significant difference between two groups.
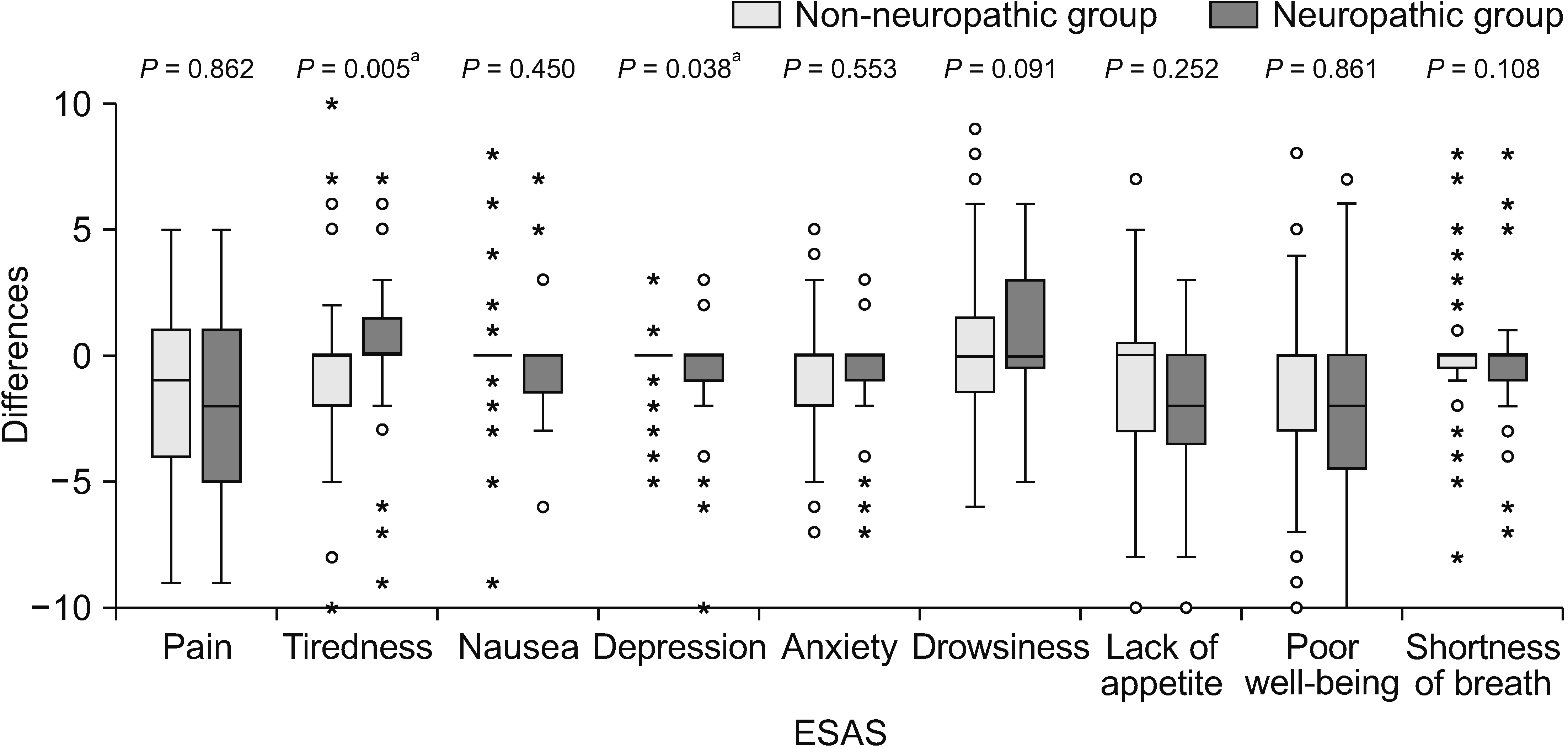
Table 1
Demographics and patient characteristics
Table 2
Summary of analgesic medications at the initial assessment
Table 3
Sensitivity, specificity, predictive values and likelihood ratios of each item of DN4 questionnaire compared with total DN4 score
Table 4
Summary of BPI scores in study patients at baseline and at week 4
Table 5
Summary of ESAS scores and treatment-related outcomes in study patients at baseline and at week 4
Table 6
Univariate analysis of risk factors for neuropathic pain features in study patients
Table 7
Multivariate analysis of risk factors for neuropathic pain features in study patients
Table 8
Summary of pain treatment at week 4




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download