Abstract
Background
This study aimed to compare the effectiveness of the pericapsular nerve group (PENG) block and intra-articular injection (IAI) of steroid-bupivacaine in the treatment of hip osteoarthritis (OA).
Methods
After randomization, patients received either a PENG block or IAI under ultrasound-guidance. Clinical evaluations were recorded at baseline, day 1, and weeks 1, 4, and 8 post-intervention. The numerical rating scale (NRS), Western Ontario and McMaster Universities Arthritis Index (WOMAC), Harris Hip Scale (HHS) scores, pain medication use determined by a quantitative analgesic questionnaire, and patient satisfaction were evaluated.
Results
Sixty patients were included in this study. NRS scores improved significantly for both groups during the follow-up compared to pretreatment (P < 0.001), with better pain scores for the PENG group (P < 0.001) at day 1 with larger effect size (Cohen’s d = 4.62), and IAI group at 4 (Cohen’s d = 5.15) and 8 (Cohen’s d = 4.33) weeks (P < 0.001). There was no significant difference in pain medication consumption (P = 0.499) and patient satisfaction (P = 0.138) between groups. Patients in the IAI group experienced significant improvement in HHS (Cohen’s d = 2.16, P = 0.007) and WOMAC (Cohen’s d = 1.02, P = 0.036) scores at 8 weeks compared to the PENG group.
Hip osteoarthritis (OA) is a chronic condition characterized by structural damage of the entire joint including inflammation of the synovial membrane, progressive erosion of the articular hyaline cartilage, and degeneration of the joint capsule and musculotendinous structures [1]. The incidence of symptomatic hip OA is estimated to be about 25% of life, and its prevalence is increasing due to the aging of the global population and the rise in obesity [2,3]. Although pain is the predominant clinical presentation, it also often severely affects daily activities and leads to societal cost [4].
Hip OA management primarily focuses on rapid symptom control including pain alleviation and functional improvement. The treatment approach for hip OA relies on a combination of non-pharmacological and pharmacological therapies consisting of lifestyle modification, physical therapy, and non-steroidal anti-inflammatory drugs [5]. Primary total hip arthroplasty is an intervention reserved for cases that do not respond efficiently to appropriate approaches for a reasonable period of time. However, even after a properly performed total joint replacement, up to 38% of patients have permanent disability and persistent postsurgical pain after 1 year [6,7]. Since implant longevity in the younger population is a contentious topic, and complications due to older age or comorbidities, non-operative treatment options are of interest and are being explored in hip OA.
Intra-articular injection (IAI) of corticosteroids, as a mainstay of non-operative treatment, has been a frequently performed procedure over the past several decades [8]. The American College of Rheumatology (ACR) and the American Association of Orthopedic Surgeons recommended IAI for the treatment of hip OA [9,10]. However, after treatment with IAIs, an increased risk of adverse joint manifestations and accelerated OA progression was found [11].
With the increasing use of ultrasound technology, peripheral nerve blocks are used as an alternative treatment approach for OA [12–14]. The pericapsular nerve group (PENG) block is an interfascial plane block for the blockade of sensory nerve branches including the articular branches of the femoral (FN), obturator (ON), and accessory obturator (AON) nerves to the anterior hip capsule, with the limitation of quadriceps or adductor muscle weakness. Then the use of the PENG block was expanded by applying it for the management of hip OA [15–17].
The primary aim of this research was to compare the outcomes of intra-articular hip injection versus PENG block on pain scores in patients with hip OA. The secondary objectives were to compare the effectiveness of intra-articular hip injection versus PENG block on functional disability, analgesic consumption, and patient satisfaction in patients suffering from hip OA.
This prospective, double-blind, randomized controlled research was approved by the local ethics committee of Diskapi Yildirim Beyazit Training and Research Hospital (decision number 84/18-2021) and subsequently registered at ClinicalTrials.gov (Registration No. NCT05136248). This trial was designed in accordance with the CONSORT guideline and written informed consent was collected from all patients. The design and process of the study were indicated in Fig. 1.
This study included 60 patients with symptomatic unilateral hip OA who were unresponsive to conservative management, including medical and physical therapy. The diagnosis was made on clinical history, examination, and radiological evaluation suggested by the ACR [18]. Participants were recruited between April and August 2021. Patients were included according to the following inclusion criteria: hip pain with a score ≥ 4 on a numerical rating scale (NRS) of 0–10 with a duration of at least six weeks, radiologic OA, grade II-III based on the Kellgren–Lawrence scale, and no abnormalities in laboratory parameters. The exclusion criteria were; hip pain due to other medical conditions, lumbar radiculopathy, rheumatological diseases, systemic active infections, malignancies, history of surgery on the affected hip, history of traumatic hip injury, history of bleeding disorders, platelet values < 150.000/µL, hip injection within the three months, and psychiatric or neurological illness.
An independent researcher who was not involved in the interventions or evaluations conducted the randomization sequence. The randomization process was concealed from the patients and the investigator who performed all evaluations during the study period. Sixty study subjects were randomly divided into two groups using the numbered envelope method. Group 1 and group 2 notes were placed separately in sealed envelopes, and each patient randomly selected an envelope and gave it to the interventionist. The interventionist opened the sealed envelope to reveal the treatment assignment for the patient just before the procedure. A single nurse who did not participate in this study prepared the injectate and assisted the interventionist during the procedure. Only the treating physician and the nurse were aware of the procedure. Patients were blinded to treatment allocation. The patients did not know which probe was used with the PENG block or the IAI,and therefore they did not know which treatment group they were in. Both techniques were performed in the operation room, in the supine position at the same level, and using the same volume of injectate. One author, blinded to group allocation and not involved in the treatments, performed outcome measurements.
A standardized ultrasonographic examination of the anterior hip was performed before the interventions in the examination room by an independent researcher. During this examination, patients were lying supine with their legs straight and hips in 15°–20° external rotation. A high frequency (7–12 MHz) linear transducer was placed on the skin in the direction of the neck and head of the femur. Both the depth and focus of the image were modified for the hip joint position. The hip joint was scanned in an anterior longitudinal and oblique view. For each patient, measurements for detecting effusion and scorings for femoral head deformity on an image on the monitor were made. Effusion was assessed by recording the bone-capsule distance in the longitudinal plane of the neck of the femur at the anterior joint recess. The bone-capsule distance is the longest ultrasonically measured intra-articular distance between the lower border of the capsule and the cortical surface of the neck of the femur. A bone-capsule distance increase of 7 mm or more or 1 mm difference between bilateral sides is accepted as intracapsular effusion in the hip joint [19]. Femoral head deformation was assessed using a rating system for the contour of the femoral head and rated as normal (round), mild (slightly flattened, still with visible curvature but an abnormally large radius), moderate (very flattened, no visible curvature of the caput), or severe (no distinct contour or femoral head identifiable) [20] (Fig. 2).
With the patient supine, a convex transducer, providing a wider field of view as well as the relative depth of the target, was initially placed on the anterior superior iliac spine (ASIS) in the horizontal plane (Fig. 3A, B). The inguinal ligament extends from the ASIS of the ilium to the tuberculum pubicum. Neurovascular structures and muscles run between the inguinal ligament and the hip. Once the ASIS was identified, the probe was slid medially and caudally along the axis until reaching the anterior inferior iliac spine (AIIS) (Fig. 3C, D), then the probe was rotated clockwise (on the right side) for approximately 45° parallel to the inguinal crease aligned with the ramus pubis to visualize the iliopubic eminence (IPE) [15]. The target for the PENG block is the musculo-fascial plane between the psoas tendon anteriorly and the pubic ramus posteriorly. In this view, between the AIIS and the IPE, where the articular branches of the FN and AON are consistently found, there is a shallow groove over which the hyperechoic psoas component of the iliopsoas muscle passes. Superior and lateral to the psoas muscle is the hypoechoic iliacus muscle, the other component of the iliopsoas muscle. Before needle insertion, applying pressure may improve the view of the hyperechoic surface of the iliopsoas notch and hyperechoic psoas tendon. Also, the pectineus muscle as well as the femoral artery and nerve were visualized superomedially in this view (Fig. 3E, F). A 27-gauge needle was used for local anesthesia with 2 mL of 1% lidocaine, and a 22-gauge, 100-mm needle was then advanced from lateral to medial with an in-plane approach, and a 13 mL solution of bupivacaine 0.25% (11 mL) and dexamethasone 8 mg (2 mL) was applied between the psoas tendon and the ramus pubis (Fig. 4). The distribution of the drug in this plane was observed.
All blocks were performed by one practitioner and all treatments were performed as an outpatient procedure. Patients were positioned supine, with the lower extremity in the neutral position. A high frequency linear transducer was placed in the anterior oblique sagittal plane, parallel to the femoral neck (Fig. 5). After visualization of the femoral head-neck junction, a 27-gauge needle was used for local anesthesia with 1% lidocaine, and then a 22-gauge, 100-mm needle was inserted into the joint capsule with continuous visualization. The same mixture, 13 mL solution of bupivacaine 0.25% (11 mL) and dexamethasone 8 mg (2 mL) was administered and visualized, filling the joint capsule.
Baseline descriptive data obtained were age, sex, and body mass index. The primary outcome of this study included the pain score at 1 month, measured by the NRS. Secondary outcome measures were NRS pain scores at day 1, week 1, and week 8 after study intervention, hip function (Western Ontario and McMaster Universities Osteoarthritis Index score [WOMAC]; The Harris Hip Score [HHS]) at weeks 4 and 8, patient satisfaction, and pain medication consumption (quantitative analgesic questionnaire [QAQ]) at week 8.
The NRS is a widely used scale to measure the intensity of a patient’s pain. Subjects were asked to score their pain on a scale of 0 to 10, with 0 indicating ‘no pain’ and 10 indicating ‘unbearable pain’. It has been shown that the NRS has a strong correlation with descriptive scales and has high sensitivity and reliability.
The WOMAC is a patient-based condition specific questionnaire consisting of 24 items divided into subscales: pain (five questions), stiffness (two questions), and physical function (17 questions). The WOMAC is used for patients suffering from hip or knee OA and is recommended as one of the highest-performing outcome measures [21]. The HHS is a multidimensional assessment that includes pain, absence of deformity, gait function, and range of motion in the hip joint. A maximum score of 100 points indicates the best function and no pain. < 70 is accepted as poor, 70–80 is fair, 80–90 is good, and 90–100 is an excellent result [22].
Mean change in analgesic consumption is evaluated using QAQ, a tool designed to obtain analgesic use reported by patients, create scores to assess and compare it and track the mean changes in analgesic drug consumption. A higher QAQ score shows higher pain medication consumption [23]. The central sensitization inventory is a self-reported outcome measure consisting of 25 multiple choice questions designed to assess symptoms regarding central sensitization to pain. A score of 40 or more points is considered a higher probability of central sensitivity syndromes or central sensitization [24]. Changes in patient satisfaction were measured using a 5-point Likert scale [25].
Sample size determination was done by using the G*Power tool version 3.1. (Heinrich-Heine-Universität) and according to the outcomes of a previous trial [26]. The NRS score one month post-IAI was 3.89 ± 1.39 in this study. Since the NRS at one month was the primary outcome, a sample size of 30 participants for each group was needed to identify a 20% difference, an α level of 0.05, and a power of 80%.
Statistical analyses were performed using the IBM SPSS version 16.0 statistical analysis program. The Shapiro–Wilk test was used to test the assumption of normality. Continuous variables, normally distributed, were presented as mean and standard deviation, and continuous variables without normal distribution as median (interquartile range). Either the independent sample t-test or Mann–Whitney U-test was used for the comparison of continuous variables. A two-way repeated measures analysis of variance (ANOVA), with group as the between-subjects factor and time (between baseline and follow-up assessments) as the within subjects factor, was used to detect any significant differences in outcome measure scores within and between the groups, with post-hoc Bonferroni tests for multiple comparisons. Cohen’s d effect size was performed to calculate the therapeutic effect for both treatment groups. Categorical data were presented as counts and percentages, and compared using chi-square and Fisher's exact tests. A value of P < 0.05 was considered as statistically significant.
A total of 60 patients eventually completed the research. No significant differences existed between the groups in terms of sex, age, body mass index, or symptom duration (P > 0.05). The baseline demographic data were similar in both groups (Table 1).
Patients in the IAI group had achieved lower NRS scores (mean score = 2.60 ± 0.67, mean difference = –4.36, Cohen’s d = 5.15) compared to patients in the PENG group (mean score = 3.50 ± 1.00, mean difference = –3.68, Cohen’s d = 3.57) which was statistically significant between the groups at week 4 (P < 0.001). In both groups, mean pain scores were significantly lowered during the 8-week follow-up period compared to the baseline values (P < 0.001) (Table 2, Fig. 6). When the groups were compared, the NRS scores in the PENG group (mean score = 2.57 ± 0.93, mean difference = –4.61, Cohen’s d = 4.62) showed significantly improved pain alleviation compared to the IAI group (mean score = 4.80 ± 0.99, mean difference = –2.16, Cohen’s d = 2.18) (P < 0.001). The pain scores between the PENG group (mean score = 2.73 ± 0.64, mean difference = –4.45, Cohen’s d = 5.08) andthe IAI group(mean score = 2.56 ± 0.62, mean difference = –4.40, Cohen’s d = 5.32) at week 1 were not different (P = 0.312). At 8 weeks post-procedure, the IAI group demonstrated better scores (mean score = 3.10 ± 0.78, mean difference = –3.86, Cohen’s d = 4.33) compared to the PENG group(mean score = 4.80 ± 1.03, mean difference = –2.38, Cohen’s d = 2.27) with a significant difference between the groups (P < 0.001).
Both groups showed significant improvements in WOMAC scores during the 8 week follow-up period compared with the baseline scores (P < 0.001). The WOMAC score was lower in the IAI group (mean score = 41.70 ± 10.35, mean difference = –10.03) with a large effect size (Cohen’s d = 1.02) compared to the PENG group (mean score = 46.93 ± 8.39, mean difference = –2.07) with a small effect size (Cohen’s d = 0.25) at 8 weeks, which was significant between the groups (P = 0.036) (Table 3).
Within both groups, the HHS scores significantly improved at each time point compared to pre-treatment (P < 0.001). Subjects in the IAI group experienced a greater reduction in HHS scores compared to subjects in the PENG group at week 4 (P = 0.021) and week 8 (P = 0.007) (Table 3).
According to the Likert scale, 70% and 62.5% of the subjects were very satisfied and satisfied in the IAI and PENG group, respectively. In both groups, there was a reduction of pain medication consumption at 8 weeks compared to the baseline (P < 0.001). However, no significant difference in patient satisfaction and QAQ scores between the IAI and PENG group was seen (Table 3).
This research was designed to investigate the actual potential value of PENG block on outcome measures including pain and function in hip OA, compared to IAI. According to many studies, IAI provide a therapeutic window of up to 8–12 weeks; therefore, a follow-up period of 8 weeks was chosen [8]. The present findings suggest that the ultrasound-guided PENG block is an effective treatment approach in patients with OA; the effect was greatest at day 1, and clinically significant relief of hip pain was sustained for 2 months. Although no significant difference was present between these two therapeutic groups when evaluating outcomes at 1 week, the IAI yielded superior pain relief at 4 and 8 weeks after the procedure compared with the PENG block.
Severe pain and loss of function are the most common symptoms of hip OA [1,4]. Driven by the impact of this chronic and debilitating condition, an array of research is being performed to diagnose OA earlier and prevent its progression. Preventative strategies for OA appear to be in their infancy and the mainstays of treatment remain symptom relief including pain reduction and improvement in functional abilities [27]. Total hip replacement is an effective treatment and is conventionally performed for patients unresponsive to conservative treatment modalities, but the risks associated with it, including the concerning failure rate (5%–15%), the limited lifespan of prostheses for young patients, and complications in many patients because of their older age or comorbidities mean that this surgery is not an appropriate option for all patients [28–30].
There is growing evidence in the literature that nerve blocks with local anesthetics (LAs) and corticosteroids are being offered for therapeutic purposes to patients with chronic pain. Ming et al. [12] demonstrated that the adductor canal block provides pain relief which improves functionality and quality of life up to three months in knee OA. Another study demonstrated that ultrasound-guided genicular nerve blockage provided significant improvements in WOMAC, NRS, and Oxford Knee Score over 3 months [13]. In a study of patients with hip OA, a significant improvement in the intensity of hip joint pain was reported for 3 months following the blockage of articular branches of the ON and FN with lidocaine and betamethasone [14].
What obviously grabs the interest of clinicians in the use of nerve blocks are the impacts on treatment outcomes in pain conditions. LAs have the potential to decrease peripheral sensitization by affecting pain nociceptors and neurons, and provide a long-lasting anti-inflammatory effect by interrupting both sympathetic postganglionic and C fibers [31]. Herein, dexamethasone was added to LA mainly as an adjuvant, and assuming a therapeutic effect related to the inflammatory component of OA. Corticosteroids can reduce pain through a combination of modulation of the inflammatory response and nerve conduction, as well as inhibition of ectopic neural discharges. In addition, corticosteroids can change the viscosity of synovial fluid and hyaluronic acid [32,33].
In the PENG block, the anterior articular capsule-sensitive branches of the FN, ON, and AON are targeted. Volume is one of the most important components affecting LA distribution and cadaver studies were performed to address this question regarding the PENG block [34]. Anatomic cadaver studies showed that the injectate spread through the bursal space between the iliopsoas and anterior hip joint capsule, reaching into the joint space. These findings verify the demonstrated effect of the PENG block, which not only targets the sensory articular branches of the hip, but also extends into the joint space [34,35]. In a case report, the PENG block was given with 20 mL 0.25% bupivacaine and 8 mg dexamethasone, and provided pain relief for 2 weeks in chronic hip pain due to OA [36]. In addition, some anecdotal cases have been reported on the effective use of pulsed radiofrequency of PENG and the neurolytic PENG block for chronic pain treatment [16,17,36,37]. According to the present results, there was a significant effect from the PENG block for two months, in addition to the greatest effect occurring on day one due to the nerve blockade with LA that was present in the PENG block.
IAIs are effectively used in the management of hip OA to relieve pain and improve functional ability, and they are recommended in symptomatic hip OA by most international guidelines [9,10]. According to the present results, IAI with bupivacaine and corticosteroid provides pain relief on day 1, which could be explained by the effect of LAs during the acute phase, anesthetizing the articular surface of the hip joint. Furthermore, the results of this study demonstrated the benefit of a single IAI over an 8-week period for patients with hip OA. This is consistent with a wealth of literature that suggest that IAIs effectively improve hip pain up to 8 weeks for hip OA patients [38,39]. However, although IAIs are highly efficacious therapeutic strategies, many patients may need repeated injections after a variable period of time, which may cause an increased risk of a variety of complications such as the progression of OA, subchondral insufficiency fracture, accelerated osteonecrosis, and destruction of the joint with bone loss [11]. Septic arthritis following IAI is a rare but major complication associated with a mortality rate of up to 15%, and is associated with permanent joint damage in up to half of those who survive [40,41]. Some studies demonstrated an increased rate of periprosthetic joint infections with total joint replacement with prior IAI [42,43], whereas other studies reported just the opposite [44,45]. Since the current literature investigating the association between IAI preoperatively and infection risk after surgery is controversial, the authors suggest that PENG block may be a treatment option which allows patients who are inevitably candidates for surgery to manage their symptoms during the period between diagnosis and surgery.
As such, the authors believe that the PENG block, as an easier and safer regional anesthesia technique, could be an alternative treatment strategy in acute on chronic episodes and for patients who are candidates for repeated IAIs to reduce the aforementioned risks. Many patients with OA have flare-ups of symptoms with varying frequency, intensity, and duration; these recurrent fluctuations may require injections of corticosteroids and LAs. This study cannot generalize the implementation of the PENG block to all patients with hip OA who are candidates for their first IAI; however, the policy in treating these patients is to perform safer procedures in the first place. To date, no major complications such as hematoma, nerve damage, or organ damage have been reported with the PENG block [46]. Furthermore, the authors believe that the concomitant application of a PENG block may augment the efficacy of IAI therapy in short-term symptom control.
This study has a few limitations. Firstly, our patients were followed for 2 months. Generally, studies assessing the short-term effects of IAIs have followed patients for 8–12 weeks. However, the analgesic effect of the PENG block diminished after one month. Therefore, a longer follow-up period might not have affected the results of this study. Secondly, due to the absence of a placebo group, a possible placebo effect from the PENG block cannot be excluded. Thirdly, although the block-related infection rate following ultrasound-guided single-injection peripheral nerve block is extremely low; further studies on the risk of injection after the PENG block are required [47]. Although different kind of ultrasound probes were used for the ultrasound examinations and interventions, it would have been an easier and feasible alternative to use the same kind of probe. Finally, a total volume of 13 mL was used for IAI. Although total injection volumes used in hip IAI in published studies range up to 15 mL, a smaller volume could be used [48–50]. Further studies are needed to compensate for these limitations.
In conclusion, our study suggests that the PENG block can provide pain relief and functional improvement up to 2 months in patients with hip OA. Ongoing challenges in hip OA management include identifying the disease in its early stages, slowing progression, and reducing the number of major surgeries. Therefore, strategies are being developed to enable patients with severe pain and functional limitations to improve their quality of life. The PENG block could be considered as a convenient and safe alternative treatment modality in acute symptom relief, especially for patients who, for medical reasons, have to postpone their total joint arthroplasty, or undergo repeated IAI. Future studies with larger patient populations and all-around evaluations of treatment outcomes are needed to accurately explore the potential of the PENG block.
Notes
REFERENCES
1. Brandt KD, Radin EL, Dieppe PA, van de Putte L. 2006; Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 65:1261–4. DOI: 10.1136/ard.2006.058347. PMID: 16973787. PMCID: PMC1798332.
2. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. 2016; Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388:1545–602. Erratum in: Lancet 2017; 389: e1. DOI: 10.1016/S0140-6736(16)31678-6. PMID: 27733282. PMCID: PMC5055577.
3. Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, et al. 2010; One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 18:1372–9. DOI: 10.1016/j.joca.2010.08.005. PMID: 20713163. PMCID: PMC2998063.
4. Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, et al. 2013; Health economics in the field of osteoarthritis: an expert's consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 43:303–13. DOI: 10.1016/j.semarthrit.2013.07.003. PMID: 23992801.
5. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. 2014; A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 43:701–12. DOI: 10.1016/j.semarthrit.2013.11.012. PMID: 24387819.
6. Dowsey MM, Nikpour M, Dieppe P, Choong PF. 2012; Associations between pre-operative radiographic changes and outcomes after total knee joint replacement for osteoarthritis. Osteoarthritis Cartilage. 20:1095–102. DOI: 10.1016/j.joca.2012.05.015. PMID: 22800770.
7. Liu SS, Buvanendran A, Rathmell JP, Sawhney M, Bae JJ, Moric M, et al. 2012; A cross-sectional survey on prevalence and risk factors for persistent postsurgical pain 1 year after total hip and knee replacement. Reg Anesth Pain Med. 37:415–22. DOI: 10.1097/AAP.0b013e318251b688. PMID: 22660483.
8. Choueiri M, Chevalier X, Eymard F. 2021; Intraarticular corticosteroids for hip osteoarthritis: a review. Cartilage. 13(1_suppl):122S–31S. DOI: 10.1177/1947603520951634. PMID: 32815375. PMCID: PMC8808783.
9. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. 2019; OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 27:1578–89. DOI: 10.1016/j.joca.2019.06.011. PMID: 31278997.
10. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2020; 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 72:149–62. Erratum in: Arthritis Care Res (Hoboken) 2021; 73: 764. DOI: 10.1002/acr.24131. PMID: 31908149.
11. Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. 2019; Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 293:656–63. DOI: 10.1148/radiol.2019190341. PMID: 31617798.
12. Ming LH, Chin CS, Yang CT, Suhaimi A. 2022; Adductor canal block versus intra-articular steroid and lidocaine injection for knee osteoarthritis: a randomized controlled study. Korean J Pain. 35:191–201. DOI: 10.3344/kjp.2022.35.2.191. PMID: 35354682. PMCID: PMC8977207.
13. Ghai B, Kumar M, Makkar JK, Goni V. 2022; Comparison of ultrasound guided pulsed radiofrequency of genicular nerve with local anesthetic and steroid block for management of osteoarthritis knee pain. Korean J Pain. 35:183–90. DOI: 10.3344/kjp.2022.35.2.183. PMID: 35354681. PMCID: PMC8977196.
14. Yavuz F, Yasar E, Ali Taskaynatan M, Goktepe AS, Tan AK. 2013; Nerve block of articular branches of the obturator and femoral nerves for the treatment of hip joint pain. J Back Musculoskelet Rehabil. 26:79–83. DOI: 10.3233/BMR-2012-00353. PMID: 23411652.
15. Girón-Arango L, Peng PW, Chin KJ, Brull R, Perlas A. 2018; Pericapsular nerve group (PENG) block for hip fracture. Reg Anesth Pain Med. 43:859–63. DOI: 10.1097/AAP.0000000000000847. PMID: 30063657.
16. Rocha Romero A, Carvajal Valdy G, Lemus AJ. 2019; Ultrasound-guided pericapsular nerve group (PENG) hip joint phenol neurolysis for palliative pain. Can J Anaesth. 66:1270–1. DOI: 10.1007/s12630-019-01448-y. PMID: 31301017.
17. Pimenta MV, Nakamura AT, Ashmawi HA, Vieira JE, Dos San H. 2021; Ultrasound-guided pericapsular nerve group and obturator nerve phenol neurolysis for refractory inpatient hip cancer metastasis pain: a case report. Braz J Anesthesiol. doi: 10.1016/j.bjane.2021.02.037. DOI: 10.1016/j.bjane.2021.02.037. PMID: 33766686.
18. Altman R, Alarcón G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. 1991; The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 34:505–14. DOI: 10.1002/art.1780340502. PMID: 2025304.
19. Koski JM, Anttila PJ, Isomäki HA. 1989; Ultrasonography of the adult hip joint. Scand J Rheumatol. 18:113–7. DOI: 10.3109/03009748909099926. PMID: 2660254.
20. Qvistgaard E, Torp-Pedersen S, Christensen R, Bliddal H. 2006; Reproducibility and inter-reader agreement of a scoring system for ultrasound evaluation of hip osteoarthritis. Ann Rheum Dis. 65:1613–9. DOI: 10.1136/ard.2005.050690. PMID: 16728462. PMCID: PMC1798465.
21. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. 1988; Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 15:1833–40. PMID: 3068365.
22. Mahomed NN, Arndt DC, McGrory BJ, Harris WH. 2001; The Harris hip score: comparison of patient self-report with surgeon assessment. J Arthroplasty. 16:575–80. DOI: 10.1054/arth.2001.23716. PMID: 11503116.
23. Robinson-Papp J, George MC, Wongmek A, Nmashie A, Merlin JS, Ali Y, et al. 2015; The quantitative analgesic questionnaire: a tool to capture patient-reported chronic pain medication use. J Pain Symptom Manage. 50:381–6. DOI: 10.1016/j.jpainsymman.2015.03.013. PMID: 25912277. PMCID: PMC4550505.
24. Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, et al. 2013; The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 14:438–45. DOI: 10.1016/j.jpain.2012.11.012. PMID: 23490634. PMCID: PMC3644381.
25. Preedy VR, Watson RR, editors. 2010. 5-point Likert scale. Handbook of disease burdens and quality of life measures. Springer;p. 4288. DOI: 10.1007/978-0-387-78665-0_6363.
26. Atchia I, Kane D, Reed MR, Isaacs JD, Birrell F. 2011; Efficacy of a single ultrasound-guided injection for the treatment of hip osteoarthritis. Ann Rheum Dis. 70:110–6. DOI: 10.1136/ard.2009.127183. PMID: 21068096.
27. Hunter DJ, Bierma-Zeinstra S. 2019; Osteoarthritis. Lancet. 393:1745–59. DOI: 10.1016/S0140-6736(19)30417-9. PMID: 31034380.
28. Healy WL, Iorio R, Clair AJ, Pellegrini VD, Della Valle CJ, Berend KR. 2016; Complications of total hip arthroplasty: standardized list, definitions, and stratification developed by The Hip Society. Clin Orthop Relat Res. 474:357–64. DOI: 10.1007/s11999-015-4341-7. PMID: 26040966. PMCID: PMC4709292.
29. Hwang SK. 2014; Experience of complications of hip arthroplasty. Hip Pelvis. 26:207–13. DOI: 10.5371/hp.2014.26.4.207. PMID: 27536583. PMCID: PMC4971395.
30. Gundel O, Thygesen LC, Gögenur I, Ekeloef S. 2020; Postoperative mortality after a hip fracture over a 15-year period in Denmark: a national register study. Acta Orthop. 91:58–62. DOI: 10.1080/17453674.2019.1680485. PMID: 31635502. PMCID: PMC7006693. PMID: 38564c83067047f1babff86a1027056f.
31. Yanagidate F, Strichartz GR. 2007; Local anesthetics. Handb Exp Pharmacol. (177):95–127. DOI: 10.1007/978-3-540-33823-9_4. PMID: 17087121.
32. Salerno A, Hermann R. 2006; Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am. 88:1361–72. DOI: 10.2106/00004623-200606000-00029. PMID: 16757774.
33. Conrozier T, Patarin J, Mathieu P, Rinaudo M. 2016; Steroids, lidocain and ioxaglic acid modify the viscosity of hyaluronic acid: in vitro study and clinical implications. Springerplus. 5:170. DOI: 10.1186/s40064-016-1762-z. PMID: 27026867. PMCID: PMC4766165.
34. Yamak Altinpulluk E, Galluccio F, Salazar C, Espinoza K, Olea MS, Hochberg U, et al. 2020; Peng block in prosthetic hip replacement: a cadaveric radiological evaluation. J Clin Anesth. 65:109888. DOI: 10.1016/j.jclinane.2020.109888. PMID: 32447169.
35. Tran J, Agur A, Peng P. 2019; Is pericapsular nerve group (PENG) block a true pericapsular block? Reg Anesth Pain Med. 44:257. DOI: 10.1136/rapm-2018-100278. PMID: 30635511.
36. Jadon A. 2021; Pulsed radiofrequency (PRF) of pericapsular nerves group (PENG) in chronic hip pain-a case report. Turk J Anaesthesiol Reanim. 49:490–3. DOI: 10.5152/TJAR.2021.1080. PMID: 35110031. PMCID: PMC9472694.
37. Fusco P, Petroni GM, DI Carlo S, Maggiani C, Marinangeli F, Ciaschi W. 2021; PENG radiofrequency and hip chronic pain: is this the future? Minerva Anestesiol. 87:1391–3. DOI: 10.23736/S0375-9393.21.15929-2. PMID: 34337925.
38. McCabe PS, Maricar N, Parkes MJ, Felson DT, O'Neill TW. 2016; The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 24:1509–17. DOI: 10.1016/j.joca.2016.04.018. PMID: 27143362.
39. Zhong HM, Zhao GF, Lin T, Zhang XX, Li XY, Lin JF, et al. 2020; Intra-articular steroid injection for patients with hip osteoarthritis: a systematic review and meta-analysis. Biomed Res Int. 2020:6320154. DOI: 10.1155/2020/6320154. PMID: 32185212. PMCID: PMC7060863.
40. Peters RH, Rasker JJ, Jacobs JW, Prevo RL, Karthaus RP. 1992; Bacterial arthritis in a district hospital. Clin Rheumatol. 11:351–5. DOI: 10.1007/BF02207192. PMID: 1458782.
41. Kaandorp CJ, van Schaardenburg D, Krijnen P. 1999; Antibiotic prophylaxis of hematogenous bacterial arthritis. Ned Tijdschr Geneeskd. 143:1808–11. Dutch. PMID: 10526583.
42. Papavasiliou AV, Isaac DL, Marimuthu R, Skyrme A, Armitage A. 2006; Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 88:321–3. DOI: 10.1302/0301-620X.88B3.17136. PMID: 16498004.
43. Cancienne JM, Werner BC, Luetkemeyer LM, Browne JA. 2015; Does timing of previous intra-articular steroid injection affect the post-operative rate of infection in total knee arthroplasty? J Arthroplasty. 30:1879–82. DOI: 10.1016/j.arth.2015.05.027. PMID: 26071248.
44. eekumar R Sr, Venkiteswaran R, Raut V. 2007; Infection in primary hip arthroplasty after previous steroid infiltration. Int Orthop. 31:125–8. DOI: 10.1007/s00264-006-0152-5. PMID: 16804732. PMCID: PMC2267548.
45. Desai A, Ramankutty S, Board T, Raut V. 2009; Does intraarticular steroid infiltration increase the rate of infection in subsequent total knee replacements? Knee. 16:262–4. DOI: 10.1016/j.knee.2008.12.002. PMID: 19138855.
46. Del Buono R, Padua E, Pascarella G, Costa F, Tognù A, Terranova G, et al. 2021; Pericapsular nerve group block: an overview. Minerva Anestesiol. 87:458–66. DOI: 10.23736/S0375-9393.20.14798-9. PMID: 33432791.
47. Alakkad H, Naeeni A, Chan VW, Abbas S, Oh J, Ami N, et al. 2015; Infection related to ultrasound-guided single-injection peripheral nerve blockade: a decade of experience at toronto Western hospital. Reg Anesth Pain Med. 40:82–4. DOI: 10.1097/AAP.0000000000000181. PMID: 25469758.
48. Park KD, Kim TK, Bae BW, Ahn J, Lee WY, Park Y. 2015; Ultrasound guided intra-articular ketorolac versus corticosteroid injection in osteoarthritis of the hip: a retrospective comparative study. Skeletal Radiol. 44:1333–40. DOI: 10.1007/s00256-015-2174-9. PMID: 26031217.
49. Ladd LM, Keene JS, Del Rio AM, Rosas HG. 2016; Correlation between hip arthroscopy outcomes and preoperative anesthetic hip joint injections, MR arthrogram imaging findings, and patient demographic characteristics. AJR Am J Roentgenol. 207:1062–9. DOI: 10.2214/AJR.16.16383. PMID: 27533286.
50. Alpaugh K, Logan C, Upadhyaya S, Martin SD. 2016; Intra-articular anesthetic injection of the hip for confirmation of symptomatic tears of the acetabular labrum: a systematic review. Orthop J Harv Med Sch. 17:30–8.
Fig. 1
Study design and follow up. PENG: pericapsular nerve group, IAI: intra-articular injection, NRS: numerical rating scale, WOMAC: Western Ontario and McMaster University Osteoarthritis Index, HHS: Harris hip score, QAQ: quantitative analgesic questionnaire, CSI: central sensitization inventory.
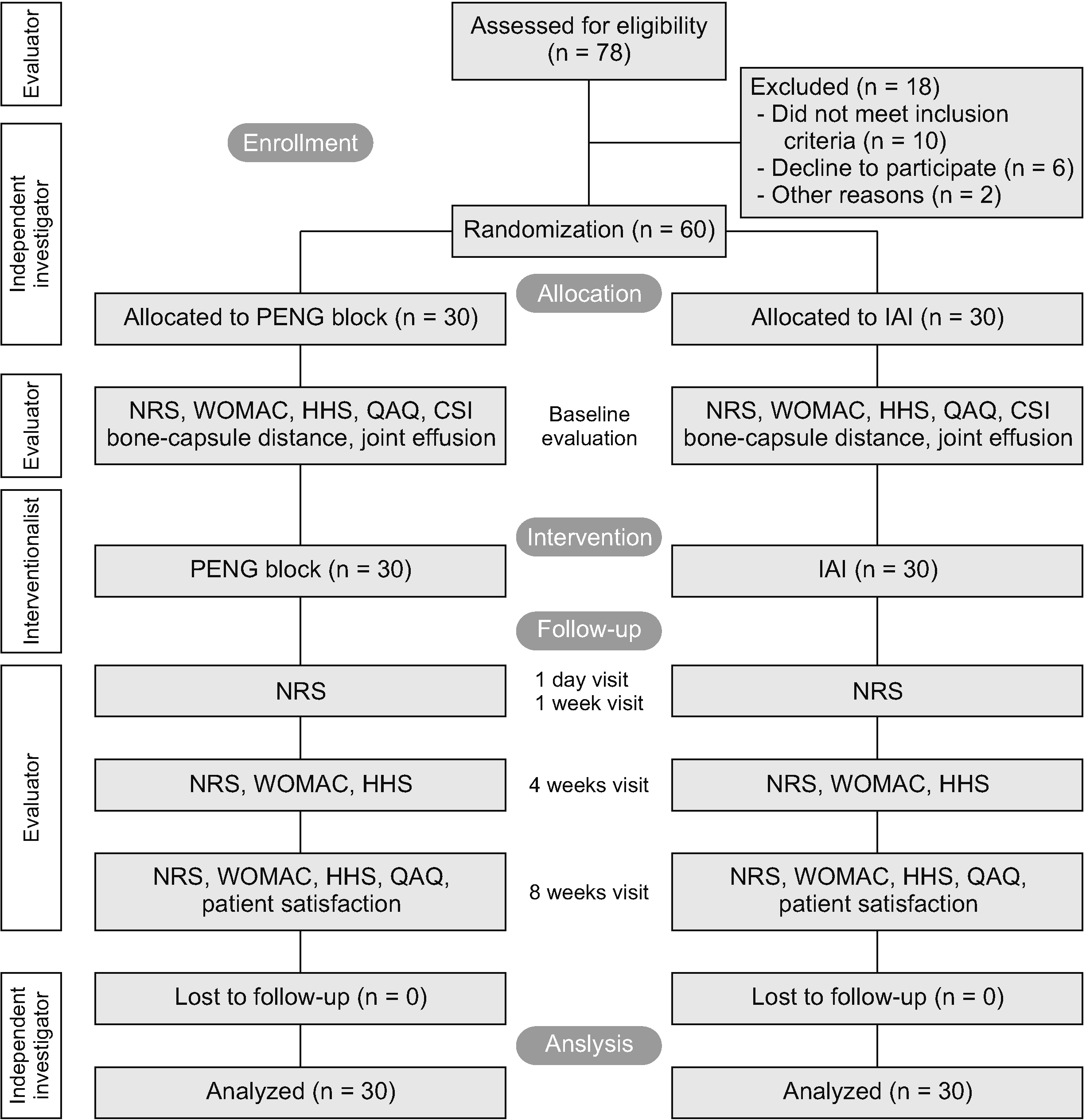
Fig. 2
Examples of femoral head score and hip joint effusion. (A) Normal: round femoral head. (B) Mild: slightly flattened. (C) Moderate: very flattened. (D) Hip effusion (11 mm). White arrow indicates bone capsule distance.
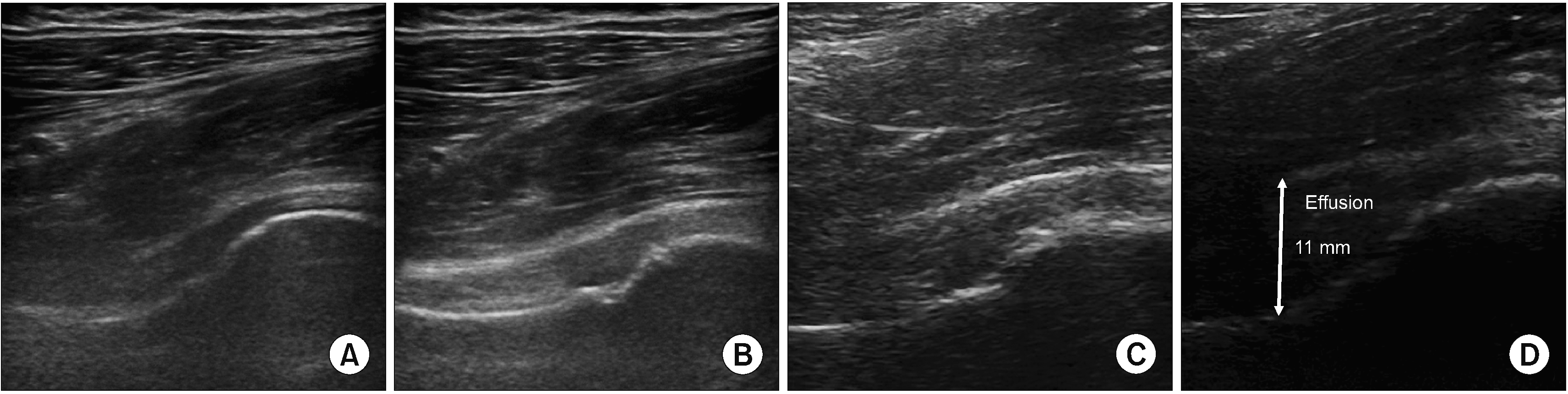
Fig. 3
Scanning technique for pericapsular nerve group block. (A, B) The ultrasound probe was placed in a transverse plane over the ASIS, (C, D) then the probe was moved medio-caudally along the axis until the AIIS, (E, F) the probe was rotated to 45° parallel to the inguinal crease aligned with the pubic ramus to visualize the iliopubic eminence. White arrows indicate the needle trajectory. ASIS: anterior superior iliac spine, AIIS: anterior inferior iliac spine, FA: femoral artery, IPE: iliopubic eminence, PT: psoas tendon.
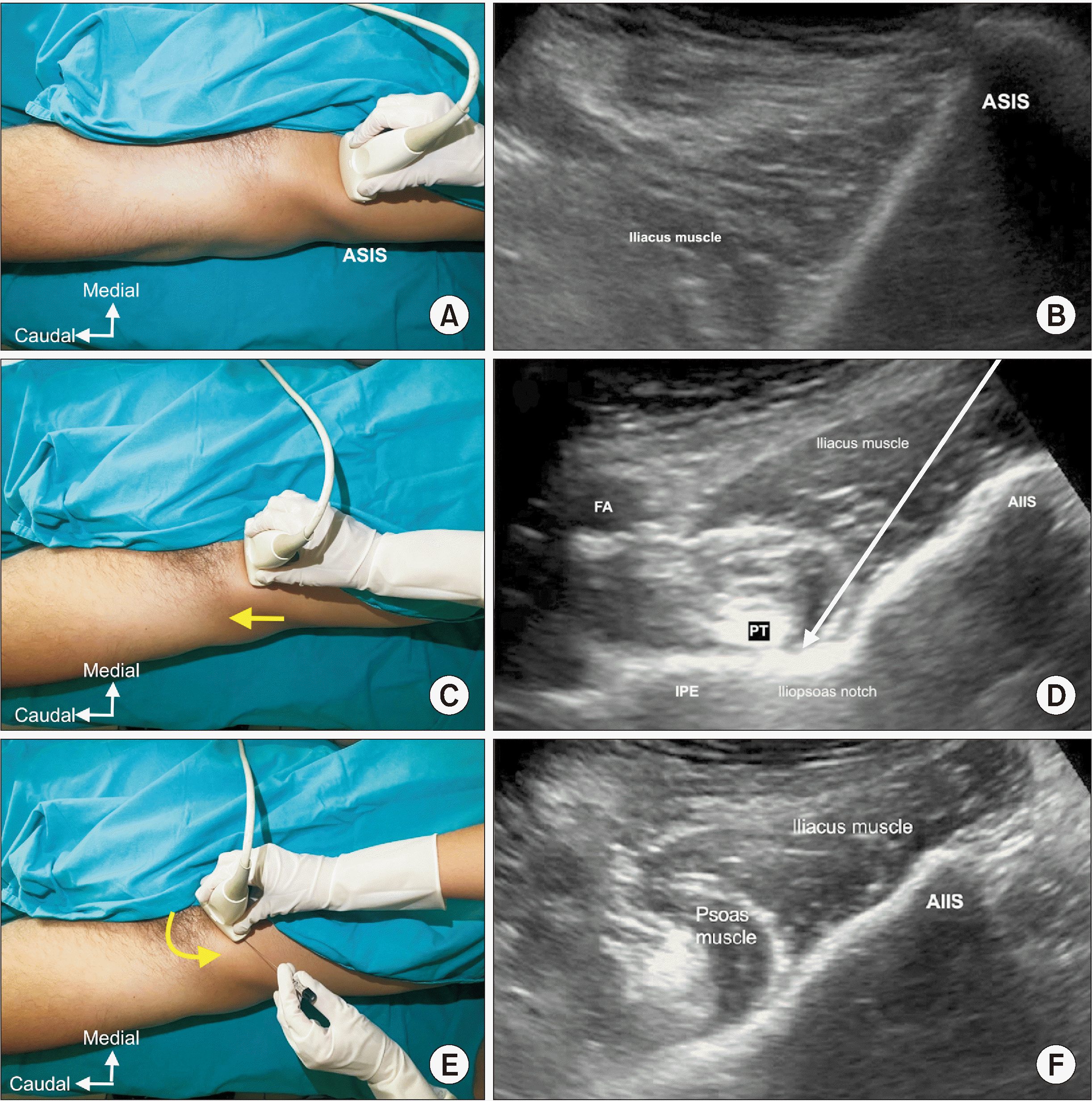
Fig. 4
(A) Transducer position, (B) sonographic image, and (C) schematic illustration showing the surrounding structures and needle position for pericapsular nerve group block. ASIS: anterior superior iliac spine, AIIS: anterior inferior iliac spine, IPE: iliopubic eminence, FA: femoral artery.
Fig. 5
Transducer placement (A) and sonoanatomy view (B) of hip-intrarticular injection. White arrows indicate the needle trajectory. Green dashed line indicates bone capsule distance. FN: femoral neck, FH: femoral head.
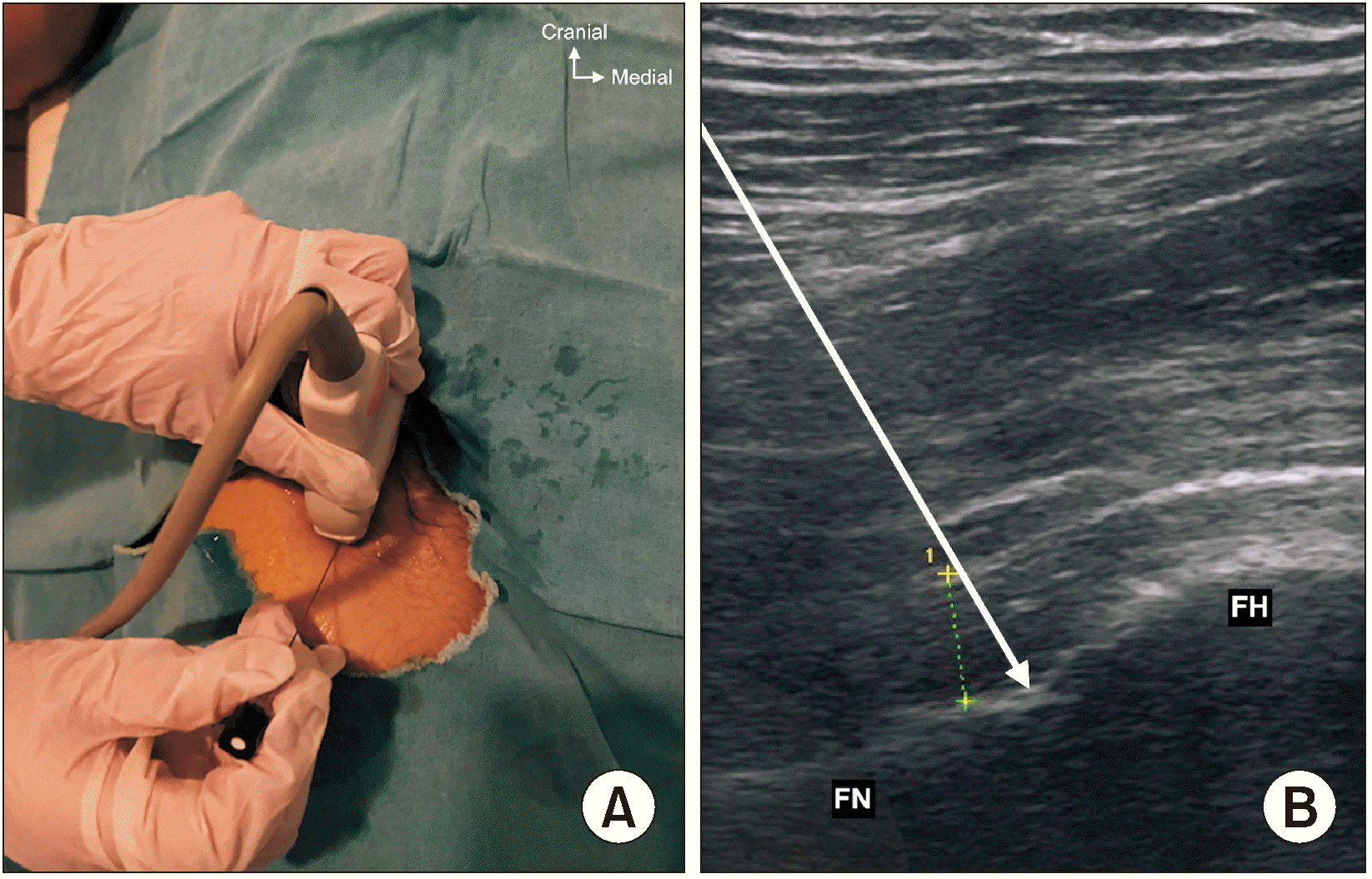
Fig. 6
Numerical rating scale (NRS) pain scores of patients. Values represent the means and standard deviations. PENG: pericapsular nerve group, IAI: intra-articular injection. *P < 0.05 vs. baseline. #P < 0.05 vs. between groups.
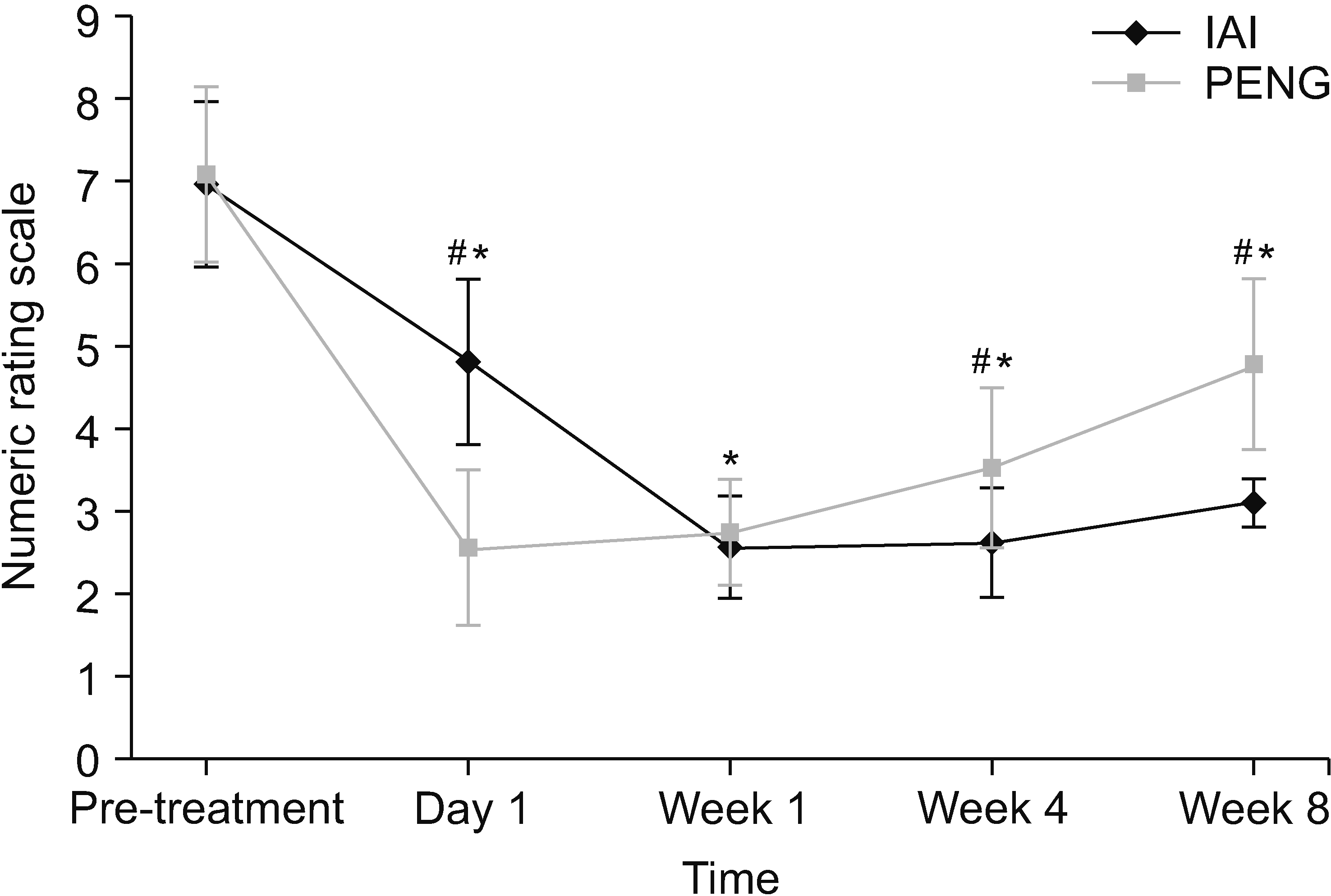
Table 1
Patients’ characteristics and baseline variables
Table 2
NRS pre- and post-injection
Table 3
Secondary outcome measures per detection time point
| Variable | IAI | PENG | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Mean score (95% CI) |
Mean difference | Cohen’s d |
Mean score (95% CI) |
Mean difference | Cohen’s d | Group comparison | |||
| WOMAC | |||||||||
| Pre-treatment |
51.73 ± 9.09 (48.41–55.06) |
49.00 ± 8.12 (46.03–51.97) |
0.225 | ||||||
| Week 4 |
42.53 ± 10.93* (38.54–46.53) |
–9.20 | 0.91 |
42.30 ± 9.13* (38.96–45.64) |
–6.70 | 0.77 | 0.929 | ||
| Week 8 |
41.70 ± 10.35* (38.04–45.60) |
–10.03 | 1.02 |
46.93 ± 8.39* (44.13–50.30) |
–2.07 | 0.25 | 0.036 | ||
| HHS | |||||||||
| Pre-treatment |
50.00 ± 6.96 (47.46–52.54) |
48.90 ± 8.11 (45.94–51.86) |
0.557 | ||||||
| Week 4 |
70.83 ± 8.71* (67.64–74.01) |
+20.83 | 2.51 |
66.13 ± 7.94* (62.67–68.52) |
17.23 | 2.14 | 0.021 | ||
| Week 8 |
67.65 ± 8.35* (64.21–70.12) |
+17.65 | 2.16 |
61.40 ± 7.60* (58.62–64.18) |
12.50 | 1.59 | 0.007 | ||
| QAQ score | |||||||||
| Pre-treatment |
3.90 ± 0.92 (3.56–4.23) |
3.53 ± 1.13 (3.11–3.94) |
0.175 | ||||||
| Week 8 |
1.73 ± 1.20* (1.29–2.17) |
–2.17 | 2.02 |
1.53 ± 1.07* (1.14–1.92) |
–2.00 | 1.81 | 0.499 | ||
| Patient satisfaction | 4.26 ± 0.86 | 3.90 ± 0.99 | 0.138 | ||||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download