INTRODUCTION
MATERIALS AND METHODS
1. Chemicals and drugs
2. Animal preparation
3. Chronic morphine tolerance model
4. Nociceptive tests
5. Surgical procedure and intra-NAc microinjection
6. Western blotting assay
7. Statistical analysis
RESULTS
1. Antinociceptive effects caused by intra-NAc microinjection of AIP into naïve rats
 | Fig. 1Antinociceptive effect of microinjection of 4, 8 or 12 µg of AIP into NAc in naïve rats. The left HWL are shown in A (noxious thermal stimulation) and C (noxious mechanical stimulation); the right HWLs are depicted in B (noxious thermal stimulation) and D (noxious mechanical stimulation). The data in the experiment are expressed as mean ± standard error of the mean. Two-way ANOVA was employed to analyze the statistical difference among experimental groups. ***P < 0.001 means relative to the control group. AIP: autocamtide-2-related inhibitory peptide, NAc: nucleus accumbens, HWL: hindpaw withdrawal latency. |
2. Effect of AIP intra-NAc administration on naïve rat CaMK II expression
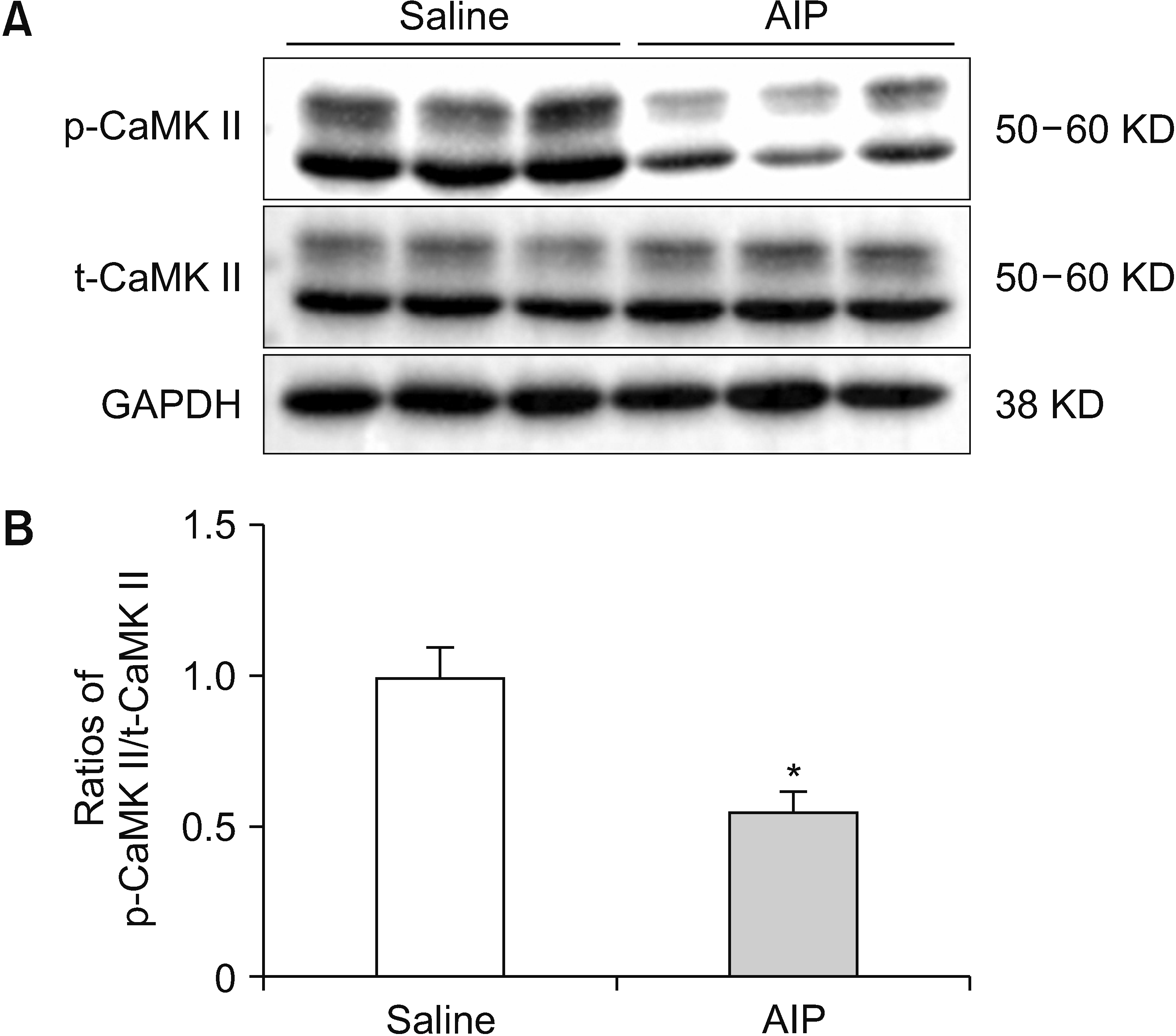 | Fig. 2Changes in CaMK II expression after AIP intra-NAc administration. (A) p-CaMK II/t-CaMK II and GAPDH western blotting results of the saline control and AIP groups. (B) p-CaMK II/t-CaMK II ratios are displayed using histograms. The data in the experiment are expressed as the mean ± standard error of the mean. Two-tailed Student’s t-test was employed to assess the statistical difference among groups. Differences with *P < 0.05 are deemed statistically significant. CaMK II: calcium/calmodulin-dependent protein kinase, AIP: autocamtide-2-related inhibitory peptide, NAc: nucleus accumbens, p-CaMK II: phosphorylated CaMK II, t-CaMK II: total CaMK II, GAPDH: glyceraldehyde-3-phosphate dehydrogenase. |
3. Influence of morphine tolerance on CaMK II expression in the NAc of rats
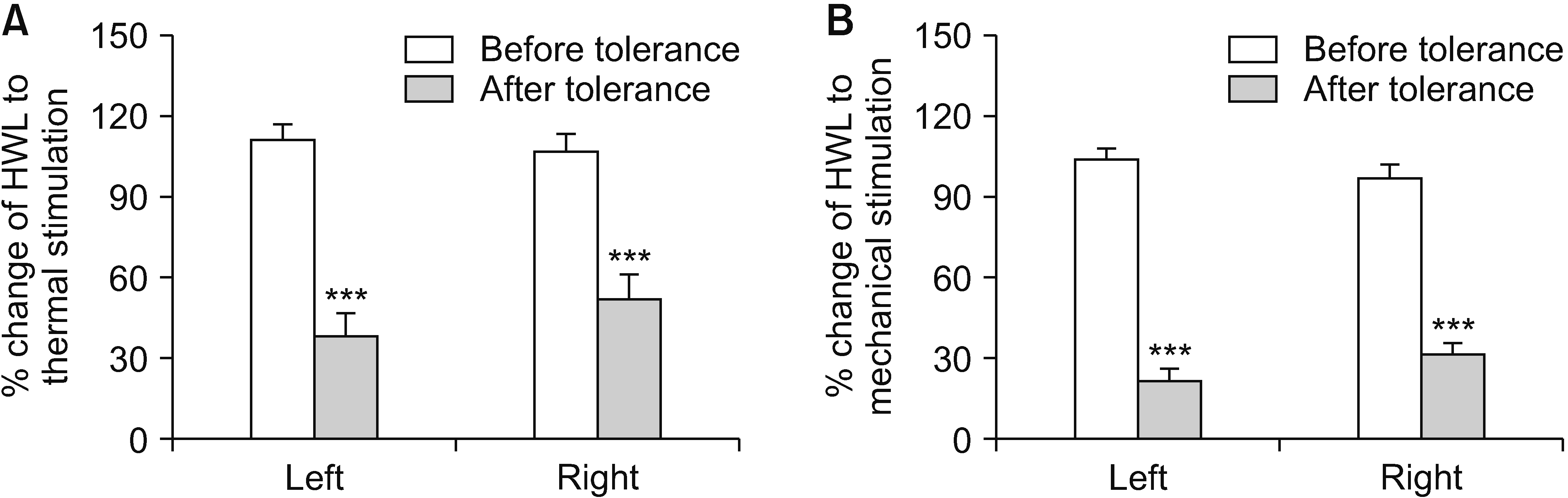 | Fig. 3Morphine tolerance induced by intraperitoneal morphine injection. A indicates HWL in response to noxious thermal stimulation; B represents HWLs to noxious mechanical stimulation. Rats have received intraperitoneal morphine injection for 7 days, and the antinociceptive effects are evaluate 30 minutes after the first morphine administration on day 1 and 7. The data of the experiment are expressed as mean ± standard error of the mean. Two-tailed Student’s t-test was employed to assess the statistical difference among groups. Differences with ***P < 0.001 are deemed statistically significant. HWL: hindpaw withdrawal latency. |
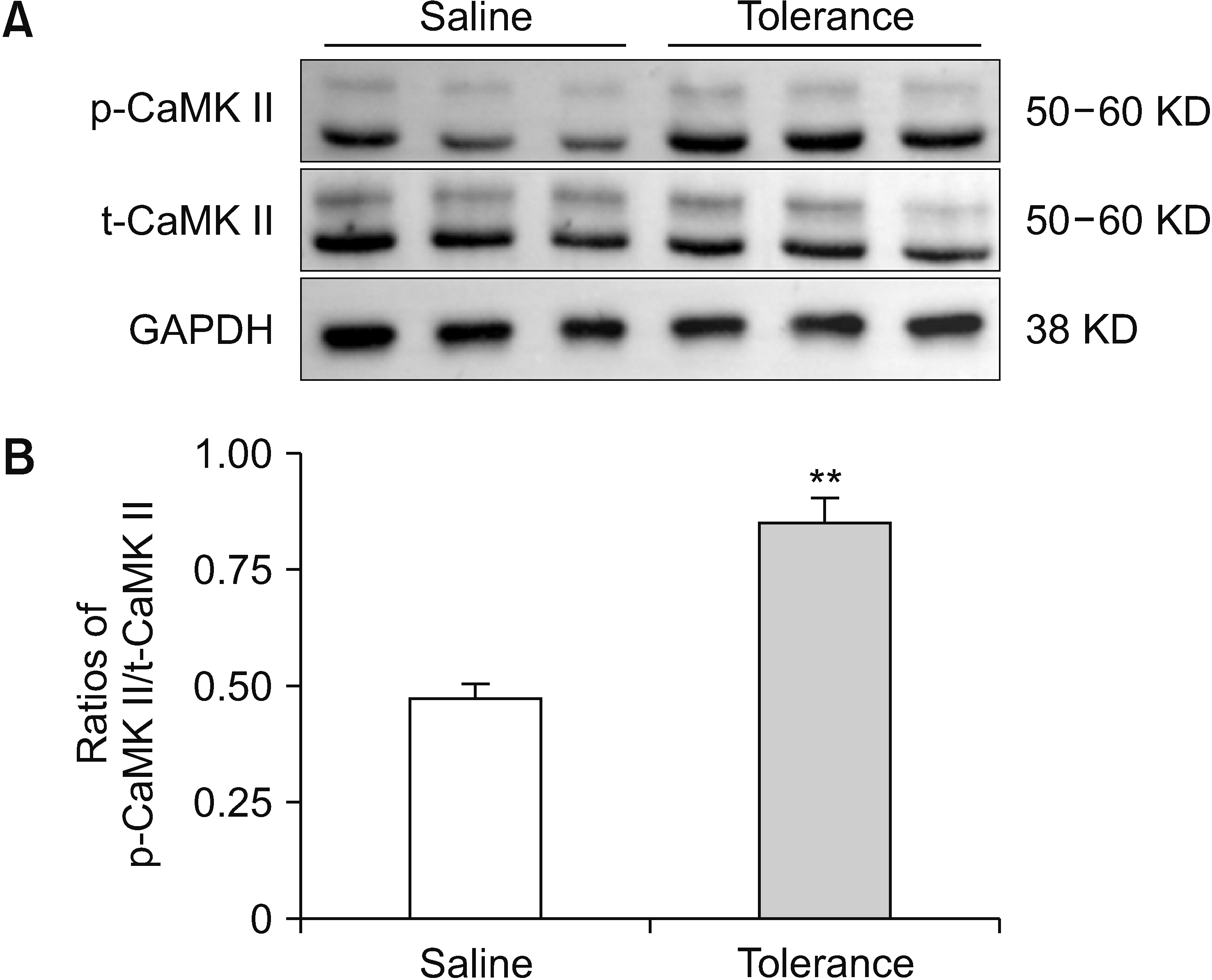 | Fig. 4Changes in CaMK II expression in morphine-tolerant rat NAc. (A) p-CaMK II/t-CaMK II and GAPDH western blotting results of the saline control and morphine tolerance groups. (B) p-CaMK II/t-CaMK II ratios are shown in histograms. The data of the experiment are expressed as mean ± standard error of the mean. Two-tailed Student’s t-test was employed to analyze statistical differences among groups. Differences with **P < 0.01 are deemed statistically significant. CaMK II: calcium/calmodulin-dependent protein kinase, NAc: nucleus accumbens, p-CaMK II: phosphorylated CaMK II, t-CaMK II: total CaMK II, GAPDH: glyceraldehyde-3-phosphate dehydrogenase. |
4. Antinociceptive effects caused by AIP intra-NAc microinjection into morphine-tolerant rats
 | Fig. 5Antinociceptive effect of microinjection of 4, 8 or 12 µg of AIP into NAc in rats showing morphine tolerance. The left HWL are depicted in A (noxious thermal stimulation) and C (noxious mechanical stimulation); the right HWL are shown in B (noxious thermal stimulation) and D (noxious mechanical stimulation). 4, 8 or 12 µg of AIP was injected into NAc 10 minutes prior to intraperitoneal injection of 10 mg/kg morphine. Time = 0 min, indicates the start of intraperitoneal morphine injection. The data of the experiment are expressed as mean ± standard error of the mean. Two-way ANOVA was employed to analyze statistical differences among groups. ***P < 0.001 means relative to the control group. AIP: autocamtide-2-related inhibitory peptide, HWL: hindpaw withdrawal latency, NAc: nucleus accumbens. |
5. Comparison of the AIP induced antinociceptive effect in the NAc of naïve and morphine-tolerant rats
 | Fig. 6Comparison of the antinociceptive effects imparted by intra-NAc microinjection of 4, 8 or 12 µg of AIP on the HWLs of naïve rats and rats showing morphine tolerance. A and B depict HWLs to noxious thermal stimulation; C and D represent HWLs to noxious mechanical stimulation. Left HWLs are presented in A and C; right HWLs are depicted in B and D. The antinociceptive effects are evaluate 30 minutes after drug injection. The data of the experiment are expressed as the mean ± standard error of the mean. Two-tailed student’s t-test was used to assess statistical differences among experimental groups. *P < 0.05, **P < 0.01 indicate compared with the control group. 4 A, 8 A or 12 A represent intra-NAc microinjection of 4, 8 or 12 µg of AIP to naïve rats; 4 A, 8 A or 12 A + morphine represent intra-NAc microinjection of 4, 8 or 12 µg of AIP to morphine-tolerant rats. AIP: autocamtide-2-related inhibitory peptide, HWL: hindpaw withdrawal latency, NAc: nucleus accumbens. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download