Abstract
Chronic postsurgical pain (CPSP) is a multifactorial condition that affects a significant proportion of patients undergoing surgery. The prevention and management of CPSP require the identification of preoperative risk factors to screen high-risk patients and establish appropriate perioperative pain management plans to prevent its development. Active postoperative pain management should be provided to prevent CPSP in patients with severe pain following surgery. These tasks have become important for perioperative team members in the management of CPSP. This review article provides a comprehensive overview of the latest research on the role of perioperative team members in preventing and managing CPSP. Additionally, it highlights practical strategies that can be employed in clinical practice, covering the definition and risk factors for CPSP, including preoperative, intraoperative, and postoperative factors, as well as a risk prediction model. The article also explores various treatments for CPSP, as well as preventive measures, including preemptive analgesia, regional anesthesia, pharmacological interventions, psychoeducational support, and surgical technique modification. This article emphasizes the importance of a comprehensive perioperative pain management plan that includes multidisciplinary interventions, using the transitional pain service as an example. By adopting a multidisciplinary and collaborative approach, perioperative team members can improve patient outcomes, enhance patient satisfaction, and reduce healthcare costs. However, further research is necessary to establish targeted interventions to effectively prevent and manage CPSP.
Chronic postsurgical pain (CPSP) has become an important problem for perioperative team members as its high incidence rate has been continuously reported [1]. It is defined as pain that persists beyond the normal healing time after surgery and can last for months or even years [2]. Its prevalence varies depending on the type of surgery, but estimates suggest that up to more than 50% of patients may experience some degree of CPSP following certain procedures, such as thoracotomy, amputation, mastectomy, and hernia repair [3]. Moreover, while CPSP prevalence has been reported mainly in North America and some European countries, recent studies have shown its high prevalence in several East Asian countries [2,4–6].
The etiologies of CPSP are complex and multifactorial and can involve a variety of patient factors, including preoperative pain, psychological comorbidities, genetic predisposition, surgical and anesthetic techniques, and perioperative management [1]. While much research has focused on identifying the risk factors for CPSP, there is growing recognition that perioperative problems, such as inadequate perioperative pain and psychological stress management, can also contribute to the development of this condition [7–10]. Therefore, perioperative team members have a critical role to play in the management of CPSP. The role of perioperative team members in the management of CPSP is multifaceted and involves a range of interventions, from preoperative patient education and risk assessment to the use of multimodal analgesia techniques and other interventional therapies. In addition, perioperative team members must be attuned to the psychological and social factors that can impact a patient's pain experience by collaborating with other departments to develop a comprehensive perioperative pain management plan.
This review article explores the latest research on the role of perioperative team members in the prevention and management of CPSP, with a focus on practical strategies that can be employed in clinical practice. Through an examination of the current evidence and best practices, this article aimed to provide perioperative team members with the knowledge and tools needed to effectively manage CPSP and improve postoperative outcomes.
Perioperative team members need to know the diagnostic criteria for CPSP because it is a common complication of surgery that can significantly impact a patient's quality of life. Understanding the diagnostic criteria for CPSP can help physicians anticipate the risk of developing this complication after surgery, accurately diagnose and manage patients who develop CPSP, and provide appropriate pain management strategies to optimize postoperative outcomes. The use of standardized diagnostic criteria is also crucial for facilitating the comparison of treatment effects in research studies.
The concept of CPSP was first proposed in 1998 by Crombie et al. [11], who attempted to determine the contribution of surgery and trauma to chronic pain. Since then, several researchers have suggested or supplemented the diagnostic criteria for CPSP [12–14], and have contributed to the creation of the latest definition of CPSP as presented in the International Classification of Diseases, 11th Revision [15] (Table 1). The following criteria have most recently been used to define CPSP: 1) chronic pain that develops or increases in intensity after a surgical procedure and persists beyond the healing process, that is, at least 3 months after surgery; 2) the pain may be localized to the surgical field, projected to the innervation territory of a nerve situated in this area, or referred to a dermatome (after surgery/injury to deep somatic or visceral tissues); 3) other causes of pain, including infection and malignancy, must be excluded, as well as pain that continues from a pre-existing pain problem; and 4) depending on the type of surgery, CPSP often has a neuropathic component [15].
It is important for perioperative team members to identify the risk factors for CPSP in managing surgical patients because these factors can help predict the likelihood of developing CPSP after surgery. By identifying high-risk patients, perioperative team members can take preventive measures and provide targeted interventions to reduce the incidence and severity of CPSP. Furthermore, understanding the risk factors can help physicians optimize pain management strategies for each patient and tailor treatment plans based on individual patient characteristics to minimize the risk of developing CPSP and improve postoperative outcomes.
Some patient factors, such as female sex, younger age, and obesity, are well-known risk factors for CPSP [1,16,17]. Another key predisposing factor for developing CPSP is preoperative pain at the site of the surgery or other parts of the body [1,3]. A multicenter study on inguinal hernia, hysterectomy, and thoracotomy showed that preoperative remote pain, as well as surgical area pain, was a significant risk factor for CPSP [18]. Patients with preoperative pain, regardless of the site, have a significantly higher risk of developing CPSP after radical prostatectomy or nephrectomy [19,20]. Chronic painful conditions in other parts of the body, such as fibromyalgia, irritable bowel syndrome, or migraines also show significant associations with CPSP development [1,3]. Furthermore, preoperative opioid use for chronic pain is likely to be closely related to the development of CPSP [21–23]. Recently, the influence of genetics on pain sensitivity and CPSP development has also been extensively investigated; however, it requires further research before it can be applied to actual clinical practice [18,24–26].
In addition to the aforementioned unmodifiable or difficult-to-modify factors, other potentially modifiable preoperative risk factors have also been discussed. Psychological factors, including anxiety, depression, catastrophizing, and general stress levels, have consistently been reported as predictors of CPSP [10,27] and are also important targets for the prevention of CPSP, which will be discussed later.
Operative factors, such as the type and duration of the surgery, extensive surgery, and extent of nerve damage, are known to be among the most important predictors of CPSP [1]. Thoracotomy, mastectomy, amputation, and orthopedic surgeries have been regarded as high-risk surgeries for CPSP development, with an incidence of approximately 50% [28,29]. Laparoscopic procedures have generally been associated with a lower incidence of CPSP compared to open procedures [1]; however, the evidence is not entirely consistent [30]. Some studies have found a significant reduction in CPSP with laparoscopic surgery, whereas others have found no difference between laparoscopic and open procedures [1,30].
The influence of anesthetic techniques on CPSP is relatively unknown. A recent multinational randomized controlled trial showed no significant intergroup difference in CPSP between a regional anesthesia-analgesia group (paravertebral block and propofol) and a general anesthesia group (inhalation anesthesia with an opioid) [31]. However, some studies predict it to be relevant. An animal study reported that general anesthetics activate a key nociceptive ion channel and enhance nerve-mediated postoperative pain [32]. Exposure to intraoperative high-dose opioids and analgesics of choice for intraoperative and postoperative analgesia may induce pain hypersensitivity via N-methyl-d-aspartate (NMDA) receptor activation [33–35]. A study that investigated CPSP 1 year after cardiac surgery showed the association between remifentanil and chronic pain in a dose-dependent manner [36]. Another study showed that the use of high-dose remifentanil with postsurgical epidural analgesia increased the incidence and extent of allodynia compared with low-dose remifentanil with presurgical epidural analgesia [37]. On the other hand, total intravenous anesthesia using propofol with remifentanil showed a lower incidence of CPSP at 3 and 6 months after thoracotomy than inhalation anesthesia using sevoflurane [38]. This can be explained by the antagonizing effect of propofol on the NMDA receptor, which may offset the effect of remifentanil-induced hyperalgesia. However, a recent randomized controlled trial in cardiac surgery showed a comparable incidence of CPSP between total intravenous anesthesia and volatile anesthesia groups [39]. Therefore, the protective effect of total intravenous anesthesia on CPSP is still inconclusive, and a well-designed, large-scale trial is required to obtain conclusive evidence.
The intensity and duration of acute postoperative pain are among the strongest and most consistent risk factors of CPSP [1,3,40,41]. The correlation between severe acute postoperative pain and CPSP has been clearly identified in various operation types, including thoracotomy, breast surgery, hernia repair, and hip arthroplasty [42–44]. Recent studies have found that patients with non-ideal acute pain trajectories after surgery were associated with a higher incidence of CPSP compared to those with ideal trajectories, which show expected improvements in postoperative pain [45,46]. If acute pain is not adequately managed, it can lead to changes in the nervous system, including sensitization of pain pathways, which can result in chronic pain that persists long after the surgical site has healed [3]. Additionally, uncontrolled acute pain can cause physical and psychological distress, contributing to the development of CPSP. Therefore, effective management of acute postoperative pain is important to prevent the development of CPSP. This may include a multimodal approach to pain management, such as using a combination of medications, nerve blocks, and non-pharmacological interventions such as cognitive therapies. In addition to the management of acute postoperative pain, other postoperative complications, reoperations, and adjuvant cancer therapies have been associated with a higher incidence of CPSP [1,47,48].
Risk prediction models for CPSP that are applicable in clinical settings may help screen high-risk patients and provide individualized preventive strategies. Several risk prediction models have been developed and validated [1]; however, a recent systematic review focused on the high risk of bias in previous models [49]. Montes et al. [18,50] developed and validated a 6-item predictive model for four surgical procedures, including hernia repair, vaginal or abdominal hysterectomy, and thoracotomy. This model includes only preoperative factors, such as surgical procedure, age, physical and mental health, and preoperative pain in the surgical field and another area with a moderate discrimination efficacy [50]. Meretoja et al. [51] developed and validated a 4-item predictive model for patients undergoing breast cancer surgery, including preoperative pain in the surgical area, high body mass index, axillary lymph node dissection, and pain intensity on postoperative day 7. Since this model contains a postoperative factor, preoperative screening with this predictive model is not possible. Recently, a predictive model was developed and validated in a prospective cohort of patients undergoing orthopedic, vascular, trauma, and general surgery [52]. The final model included four predictors: preoperative opioid use, bone surgery, pain score on postoperative day 14, and the presence of a painful cold within the painful area 2 weeks after surgery, with a reported area under the curve of 0.82. The neuropathic characteristics of pain were shown to be strong predictors of CPSP development in this model, supporting the neuropathic mechanism with nervous system sensitization [52]. In addition to the development of a more accurate prediction model for CPSP, future research is needed to determine whether CPSP can be reduced when the prediction model is used in clinical practice. Moreover, predicting the risk of CPSP can have economic benefits by enabling the targeted allocation of limited medical resources to patients in need of intensive care.
Acute postoperative pain is a major modifiable contributor to the development of CPSP and has been considered a key target for preventive interventions. Efforts to prevent CPSP typically begin with the management of acute postoperative pain. Although the underlying mechanisms are not yet fully understood, nociceptive sensitization and structural changes in the central nervous system appear to lower the mechanical threshold and exacerbate the response to noxious stimuli [3,35,53,54]. Furthermore, as perioperative team members also have a vital role in the management of acute postoperative pain, their contribution to the prevention of CPSP can be enhanced through the improvement of acute postoperative pain management.
Preemptive analgesia, which has recently been used as an option for multimodal analgesia, can prevent CPSP and reduce acute postoperative pain [55]. The concept of preemptive analgesia was first suggested by Woolf et al. [56] in 1993. This technique involves providing analgesia before surgical incision to block the noxious preoperative stimulus and prevent the activation of primary afferent nociceptors. The association between preemptive analgesia and CPSP prevention has been explored in several studies. For instance, a randomized controlled trial on thoracotomy divided 69 patients into three groups: the pre-thoracic epidural analgesia (TEA) group (epidural analgesia from preoperative to postoperative period), a post-TEA group (epidural analgesia during the postoperative period), and an intravenous patient-controlled analgesia group (postoperative intravenous analgesia) [57]. The severity of acute postoperative pain and the incidence and severity of CPSP, evaluated after 6 months, significantly decreased in the pre-TEA group compared with those in the other two groups [57]. Another study comparing pre-incisional paravertebral blocks and sham blocks in breast surgery showed a significant difference in acute postoperative pain and pain evaluated at 1, 6, and 12 months after surgery [58]. In a randomized controlled trial on phantom limb pain, the incidence of CPSP was significantly reduced by using intravenous or epidural analgesia starting 48 hours before surgery [59]. Recently, a meta-analysis reported that preemptive epidural analgesia before surgical incision reduced the incidence of CPSP after thoracotomy compared to epidural analgesia after incision [60]. Proper pain management at the right time during the perioperative period may lead to better outcomes for preventing CPSP.
The anesthetic agents used during general anesthesia may have an impact on the development of CPSP. Propofol-based anesthesia has been suggested to decrease the incidence of CPSP compared with inhalation-based anesthesia [38,61,62]. Nitrous oxide, an NMDA receptor antagonist, has also been reported to reduce CPSP [63]. However, in a follow-up study of a large-scale multicenter trial, nitrous oxide did not demonstrate any significant difference in the incidence of CPSP at 12 months after surgery, except in patients with variants in the methylenetetrahydrofolate reductase gene [64]. As high-dose opioid exposure during surgery is widely recognized as a risk factor for CPSP [65], individualized tailoring of the opioid dose with intraoperative nociception monitors may potentially be helpful in preventing CPSP [66].
Regional block techniques in various surgeries have been shown to be effective in preventing the development of CPSP. Although the mechanism is not completely understood, it appears to modulate pain signals and neuroplasticity, thereby decreasing central sensitization and pain chronification [67]. In systematic reviews comparing regional anesthesia with conventional analgesia, thoracic epidural anesthesia in thoracotomy seems to effectively reduce CPSP [68,69]. However, a recent meta-analysis has reported that thoracic paravertebral block is not effective in preventing CPSP following thoracic surgery [70]. The use of regional analgesia or local anesthetics via any route in breast cancer surgery appears to decrease the incidence of CPSP, although the level of evidence is insufficient to draw definitive conclusions [69,71]. While evidence in breast surgery generally supports the use of paravertebral block [68,69], the most recent meta-analysis indicates low-quality evidence for its beneficial effect on CPSP, specifically in chronic postsurgical neuropathic pain [72]. In a clinical study of extremity amputation, the prolonged use of peripheral nerve block catheters considerably reduced the incidence and severity of phantom limb pain [73]. Recently, several ultrasound-guided interfascial plane blocks have shown promising results in preventing CPSP [74–76]. However, the precise effects of these blocks on CPSP remain difficult to determine. Despite the limitations in the methodologies of many clinical trials, regional analgesia remains a promising strategy to prevent the development of CPSP [8,77]. Furthermore, the opioid-sparing effect of effectively controlling intraoperative and immediate postoperative pain by regional analgesia may also contribute to decreasing CPSP [78].
Aggressive pharmacological interventions to prevent CPSP seem promising; however, evidence in this field has shown somewhat disappointing results. Intraoperative ketamine appears to be beneficial for the control of acute postoperative pain; however, its impact on chronic pain remains controversial [4,28,79,80]. Recently, a large multicenter randomized controlled trial regarding the effectiveness of ketamine on CPSP has been actively conducted in Australia, and the results are expected soon [81]. Gabapentinoids, antidepressants, and local anesthetics, such as intravenous and topical lidocaine, have also been reported to be effective in reducing CPSP [28]. However, the most recent meta-analysis concluded that the effects of these drugs were smaller than expected, and their clinical implications were unclear [82].
Although pharmacological approaches have been the mainstay of interventions to prevent CPSP, the aspect of psychoeducation has recently been focused on. As previously described, preoperative psychological problems are a consistent risk factor for CPSP. In this regard, providing accurate information to patients and lowering anxiety levels through preoperative psychoeducation may help prevent the progression to chronic pain [83]. One meta-analysis, which included 15 randomized controlled trials, reported that active perioperative psychotherapy reduced the severity of CPSP and improved physical impairment [84]. Psychoeducational interventions have been an important component of the multimodal approach in the transitional pain service (TPS) at Toronto General Hospital throughout the perioperative period, which has been shown to decrease CPSP and opioid consumption [85].
Operative risk factors, such as the type of surgery and the duration of the procedure, are critical but difficult-to-modify factors that can affect the development of CPSP. Less invasive surgeries are expected to cause less chronic pain because nerve injury caused by dissection or retraction of the tissue is a crucial risk factor for CPSP [86]. In this regard, laparoscopic surgeries, such as herniorrhaphy or cholecystectomy, have shown less progression to CPSP than open surgery [1]. However, in a study comparing thoracotomy and video-assisted thoracic surgery (VATS), the two groups did not show significant differences in CPSP-related outcomes 1 year after the operation [87]. A nationwide questionnaire study also showed a comparable incidence of CPSP between patients undergoing VATS and thoracotomy [88], possibly due to intercostal nerve damage occurring in both groups, which is a significant risk factor for chronic neuropathic pain [89]. However, with the evolution of operational techniques, a recent study showed that single-port VATS, with a smaller surgical incision, reduced the incidence of CPSP compared with multi-port VATS [90].
The treatment of established CPSP has been addressed relatively less than its prevention. However, considering its high prevalence and clinical impact on quality of life after surgery, its treatment should also be considered an important issue among perioperative team members.
CPSP, diagnosed after excluding other causes of pain, can be treated with various pharmacological and non-pharmacological treatments, similar to other chronic pain conditions. As in the recently proposed modified World Health Organization analgesic ladder for chronic non-cancer pain, the intensity of analgesic medications can be upgraded step-by-step depending on the patient's response to treatment, starting with a non-opioid analgesic method [91]. Strong opioids should be used carefully in a limited number of patients in the last step, weighing their benefits and risks [92]. In addition, since the prevalence of the neuropathic component in CPSP has been known to be high [93,94], various pharmacologic and interventional treatments used for neuropathic pain can be useful in the treatment of CPSP [95]. A recently reported comprehensive algorithm for neuropathic pain has suggested non-opioid medications such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, gabapentinoids, topicals, and transdermal substances as first-line therapy and tramadol and tapentadol as second-line therapy [96]. Moreover, their effects have also been reported in patients with CPSP [97]. Psychological interventions are also considered an important option in the treatment of CPSP because psychological factors have been consistently reported as important risk factors for CPSP [21]. According to a systematic review, preliminary evidence suggests that psychological interventions based on cognitive behavioral therapy can reduce the intensity and disability associated with CPSP [98]. Furthermore, the effectiveness of neuromodulation techniques, such as radiofrequency ablation and spinal cord stimulation, has been reported in various surgeries [99–106]. Considering the neuropathic component underlying CPSP, neuromodulation is expected to be an important therapeutic option for patients who are non-responsive to pharmacological interventions.
However, there is still a lack of evidence regarding the treatment of CPSP. To the best of the authors’ knowledge, only one systematic review has reported on this issue thus far [97]. Unfortunately, a meta-analysis was not conducted because of the heterogeneity of the study [97]. Therefore, the authors concluded that all interventions had insufficient evidence to support the conclusion of their effectiveness. To improve the management of CPSP, robust evidence should be provided by conducting well-designed studies on this issue, as well as researching the prediction and prevention of CPSP in the future.
The TPS, launched in 2014 at the Toronto General Hospital, provides comprehensive perioperative pain management from the preoperative to the post-discharge phase [85]. Its main goal is to minimize the transition of acute postoperative pain to CPSP. To this end, TPSs provide multidisciplinary pain management in patients whose acute pain is likely to be uncontrolled after surgery or in patients with a high possibility of developing CPSP preoperatively.
TPS can be divided into three stages depending on the timing of the intervention (preoperative, immediate postoperative in-hospital, and postoperative in outpatient settings) [85]. First, the risk of developing CPSP is assessed preoperatively, and a personalized perioperative pain management plan is established based on the level of risk. In addition, psychological consultation and patient and caregiver education can be provided. Second, immediately after surgery, acute postoperative pain is actively controlled using a multimodal approach by a multidisciplinary pain management team. Finally, the outpatient visit schedule is adjusted by phone within 3 days of surgery, and usually, a follow-up appointment is scheduled in the outpatient clinic within 2–3 weeks after discharge. If the patient has difficulty controlling the pain, the schedule can be rescheduled. Outpatient clinics offer a comprehensive approach to pain management, which includes multidisciplinary interventions, such as psychological intervention and physiotherapy. Some studies have indicated that TPS is effective in alleviating postoperative pain and in reducing opioid use after discharge [107,108]. Recent reports also suggest that implementing TPS for various surgical patients resulted in a substantial reduction in the severity of CPSP and opioid consumption after discharge [109–112]. Moreover, implementing TPSs is expected to be economically feasible by reducing the social costs associated with CPSP, such as loss of productivity and treatment expenses [113].
However, currently, only a limited number of institutions have implemented TPS. The scarcity of resources and manpower in the medical environment may make it challenging to provide such services in the near future. Under these circumstances, pain physicians at pain centers, trained in anesthesiology and pain medicine, are expected to play a vital role in managing patients with CPSP in collaboration with perioperative team members. Pain physicians possess extensive knowledge of perioperative care, as well as expertise in treating chronic neuropathic pain, making them valuable partners in the management of CPSP. Therefore, perioperative team members should collaborate with pain physicians to provide a continuous set of treatments for patients with CPSP. This collaborative approach would allow for the provision of comprehensive care and the management of the complex needs of patients with CPSP.
As demonstrated in Fig. 1, perioperative team members can play a crucial role in managing CPSP, and should take into account its high prevalence and impact on patients' quality of life. To prevent and manage CPSP, perioperative team members should first evaluate the preoperative risk factors of each patient. For high-risk patients, perioperative team members must establish preventive interventions that offer multidisciplinary approaches, including psychoeducation, in addition to the institution's routine protocol. Second, to minimize the risk factors of CPSP, perioperative team members should establish a multimodal opioid-sparing analgesia regimen, which would reduce both acute postoperative pain severity and perioperative opioid use. Moreover, patients who experience acute postoperative pain that is not alleviated by the routine perioperative pain management protocol should receive additional treatment, including a referral to a pain specialist, within a multidisciplinary system. Finally, it is essential to establish a system that provides appropriate treatment even after discharge for high-risk patients susceptible to developing CPSP. This system should incorporate a follow-up care plan that emphasizes early identification and intervention for CPSP.
In summary, CPSP is a multifactorial and complex condition that can significantly affect the quality of life of patients undergoing surgery. The implementation of a comprehensive perioperative pain management plan that includes multidisciplinary interventions is essential to reduce the incidence and severity of CPSP. The establishment of an integrated management plan for CPSP that involves the cooperation of perioperative team members in various departments is crucial for optimizing patient outcomes and minimizing the social costs associated with this condition. Future research should focus on identifying patient-specific risk factors for CPSP and developing targeted interventions to prevent and manage CPSP effectively. By addressing CPSP using a multidisciplinary and collaborative approach, caregivers can enhance patient satisfaction, reduce healthcare costs, and improve patient outcomes after surgery.
Notes
REFERENCES
1. Rosenberger DC, Pogatzki-Zahn EM. 2022; Chronic post-surgical pain - update on incidence, risk factors and preventive treatment options. BJA Educ. 22:190–6. DOI: 10.1016/j.bjae.2021.11.008. PMID: 35496645.
2. Yoon S, Hong WP, Joo H, Kim H, Park S, Bahk JH, et al. 2020; Long-term incidence of chronic postsurgical pain after thoracic surgery for lung cancer: a 10-year single-center retrospective study. Reg Anesth Pain Med. 45:331–6. DOI: 10.1136/rapm-2020-101292. PMID: 32188682.
3. Glare P, Aubrey KR, Myles PS. 2019; Transition from acute to chronic pain after surgery. Lancet. 393:1537–46. DOI: 10.1016/S0140-6736(19)30352-6. PMID: 30983589.
4. Kang C, Cho AR, Kim KH, Lee EA, Lee HJ, Kwon JY, et al. 2020; Effects of intraoperative low-dose ketamine on persistent postsurgical pain after breast cancer surgery: a prospective, randomized, controlled, double-blind study. Pain Physician. 23:37–47. DOI: 10.36076/ppj.2020/23/37. PMID: 32013277.
5. Sugiyama Y, Iida H, Amaya F, Matsuo K, Matsuoka Y, Kojima K, et al. 2018; Prevalence of chronic postsurgical pain after thoracotomy and total knee arthroplasty: a retrospective multicenter study in Japan (Japanese Study Group of Subacute Postoperative Pain). J Anesth. 32:434–8. DOI: 10.1007/s00540-018-2481-0. PMID: 29523994.
6. Wang B, Gao P, Wang J, Zheng H. 2022; Association between aesthetic satisfaction and chronic postsurgical pain in breast cancer patients treated with one stage prosthesis implantation. Sci Rep. 12:1258. DOI: 10.1038/s41598-022-05185-z. PMID: 35075177. PMCID: PMC8786942. PMID: 124fbee6a8ae46c1bd2f4ba86b2297a3.
7. Morrison RS, Flanagan S, Fischberg D, Cintron A, Siu AL. 2009; A novel interdisciplinary analgesic program reduces pain and improves function in older adults after orthopedic surgery. J Am Geriatr Soc. 57:1–10. DOI: 10.1111/j.1532-5415.2008.02063.x. PMID: 19054187. PMCID: PMC2729399.
8. Chen YK, Boden KA, Schreiber KL. 2021; The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia. 76(Suppl 1):8–17. DOI: 10.1111/anae.15256. PMID: 33426669. PMCID: PMC8369227.
9. Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. 2014; Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 18:273–82. DOI: 10.1016/j.smrv.2013.07.002. PMID: 24074687.
10. Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. 2012; Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. 28:819–41. DOI: 10.1097/AJP.0b013e31824549d6. PMID: 22760489.
11. Crombie IK, Davies HT, Macrae WA. 1998; Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 76:167–71. DOI: 10.1016/S0304-3959(98)00038-4. PMID: 9696470.
12. Macrae WA. 2001; Chronic pain after surgery. Br J Anaesth. 87:88–98. DOI: 10.1093/bja/87.1.88. PMID: 11460816.
13. Werner MU, Kongsgaard UE. 2014; I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 113:1–4. DOI: 10.1093/bja/aeu012. PMID: 24554546.
14. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. 2015; A classification of chronic pain for ICD-11. Pain. 156:1003–7. DOI: 10.1097/j.pain.0000000000000160. PMID: 25844555. PMCID: PMC4450869.
15. Schug SA, Lavand'homme P, Barke A, Korwisi B, Rief W, Treede RD. 2019; The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 160:45–52. DOI: 10.1097/j.pain.0000000000001413. PMID: 30586070.
16. Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, et al. 2012; Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 13:1172–87. DOI: 10.1016/j.jpain.2012.09.013. PMID: 23182226. PMCID: PMC3511823.
17. van Helmond N, Timmerman H, van Dasselaar NT, van de Pol CC, Olesen SS, Drewes AM, et al. 2017; High body mass index is a potential risk factor for persistent postoperative pain after breast cancer treatment. Pain Physician. 20:E661–71. PMID: 28727711.
18. Montes A, Roca G, Sabate S, Lao JI, Navarro A, Cantillo J, et al. 2015; Genetic and clinical factors associated with chronic postsurgical pain after hernia repair, hysterectomy, and thoracotomy: a two-year multicenter cohort study. Anesthesiology. 122:1123–41. DOI: 10.1097/ALN.0000000000000611. PMID: 25985024.
19. Gerbershagen HJ, Ozgür E, Dagtekin O, Straub K, Hahn M, Heidenreich A, et al. 2009; Preoperative pain as a risk factor for chronic post-surgical pain - six month follow-up after radical prostatectomy. Eur J Pain. 13:1054–61. DOI: 10.1016/j.ejpain.2008.11.020. PMID: 19138869.
20. Gerbershagen HJ, Dagtekin O, Rothe T, Heidenreich A, Gerbershagen K, Sabatowski R, et al. 2009; Risk factors for acute and chronic postoperative pain in patients with benign and malignant renal disease after nephrectomy. Eur J Pain. 13:853–60. DOI: 10.1016/j.ejpain.2008.10.001. PMID: 19010073.
21. Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. 2017; The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 11:169–77. DOI: 10.1177/2049463717720636. PMID: 29123661. PMCID: PMC5661689.
22. Rozet I, Nishio I, Robbertze R, Rotter D, Chansky H, Hernandez AV. 2014; Prolonged opioid use after knee arthroscopy in military veterans. Anesth Analg. 119:454–9. DOI: 10.1213/ANE.0000000000000292. PMID: 24977636.
23. VanDenKerkhof EG, Hopman WM, Goldstein DH, Wilson RA, Towheed TE, Lam M, et al. 2012; Impact of perioperative pain intensity, pain qualities, and opioid use on chronic pain after surgery: a prospective cohort study. Reg Anesth Pain Med. 37:19–27. DOI: 10.1097/AAP.0b013e318237516e. PMID: 22157741.
24. Hoofwijk DM, van Reij RR, Rutten BP, Kenis G, Buhre WF, Joosten EA. 2016; Genetic polymorphisms and their association with the prevalence and severity of chronic postsurgical pain: a systematic review. Br J Anaesth. 117:708–19. DOI: 10.1093/bja/aew378. PMID: 27956669.
25. Wang L, Wei C, Xiao F, Chang X, Zhang Y. 2019; Influences of COMT rs4680 and OPRM1 rs1799971 polymorphisms on chronic postsurgical pain, acute pain, and analgesic consumption after elective cesarean delivery. Clin J Pain. 35:31–6. DOI: 10.1097/AJP.0000000000000654. PMID: 30234521.
26. van Reij RRI, Hoofwijk DMN, Rutten BPF, Weinhold L, Leber M, Joosten EAJ, et al. Italian Pain Group. 2020; The association between genome-wide polymorphisms and chronic postoperative pain: a prospective observational study. Anaesthesia. 75(Suppl 1):e111–20. DOI: 10.1111/anae.14832. PMID: 31903573. PMCID: PMC6973279.
27. Giusti EM, Lacerenza M, Manzoni GM, Castelnuovo G. 2021; Psychological and psychosocial predictors of chronic postsurgical pain: a systematic review and meta-analysis. Pain. 162:10–30. DOI: 10.1097/j.pain.0000000000001999. PMID: 32694386.
28. Humble SR, Dalton AJ, Li L. 2015; A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain. 19:451–65. DOI: 10.1002/ejp.567. PMID: 25088289. PMCID: PMC4405062.
29. Simanski CJ, Althaus A, Hoederath S, Kreutz KW, Hoederath P, Lefering R, et al. 2014; Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med. 15:1222–9. DOI: 10.1111/pme.12434. PMID: 24716774.
30. Joris JL, Georges MJ, Medjahed K, Ledoux D, Damilot G, Ramquet CC, et al. 2015; Prevalence, characteristics and risk factors of chronic postsurgical pain after laparoscopic colorectal surgery: retrospective analysis. Eur J Anaesthesiol. 32:712–7. DOI: 10.1097/EJA.0000000000000268. PMID: 26086282.
31. Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. 2019; Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 394:1807–15. DOI: 10.1016/S0140-6736(19)32313-X. PMID: 31645288.
32. Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. 2008; General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 105:8784–9. DOI: 10.1073/pnas.0711038105. PMID: 18574153. PMCID: PMC2438393.
33. Rivat C, Laulin JP, Corcuff JB, Célèrier E, Pain L, Simonnet G. 2002; Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology. 96:381–91. DOI: 10.1097/00000542-200202000-00025. PMID: 11818772.
34. Cabañero D, Campillo A, Célérier E, Romero A, Puig MM. 2009; Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology. 111:1334–45. DOI: 10.1097/ALN.0b013e3181bfab61. PMID: 19934880.
35. Richebé P, Capdevila X, Rivat C. 2018; Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 129:590–607. DOI: 10.1097/ALN.0000000000002238. PMID: 29738328.
36. van Gulik L, Ahlers SJ, van de Garde EM, Bruins P, van Boven WJ, Tibboel D, et al. 2012; Remifentanil during cardiac surgery is associated with chronic thoracic pain 1 yr after sternotomy. Br J Anaesth. 109:616–22. DOI: 10.1093/bja/aes247. PMID: 22831889.
37. Salengros JC, Huybrechts I, Ducart A, Faraoni D, Marsala C, Barvais L, et al. 2010; Different anesthetic techniques associated with different incidences of chronic post-thoracotomy pain: low-dose remifentanil plus presurgical epidural analgesia is preferable to high-dose remifentanil with postsurgical epidural analgesia. J Cardiothorac Vasc Anesth. 24:608–16. DOI: 10.1053/j.jvca.2009.10.006. PMID: 20005744.
38. Song JG, Shin JW, Lee EH, Choi DK, Bang JY, Chin JH, et al. 2012; Incidence of post-thoracotomy pain: a comparison between total intravenous anaesthesia and inhalation anaesthesia. Eur J Cardiothorac Surg. 41:1078–82. DOI: 10.1093/ejcts/ezr133. PMID: 22290901.
39. Yu H, Xu Z, Dai SH, Jiang JL, He LL, Zheng JQ, et al. 2021; The effect of propofol versus volatile anesthetics on persistent pain after cardiac surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth. 35:2438–46. DOI: 10.1053/j.jvca.2020.10.025. PMID: 33183935.
40. Steyaert A, Lavand'homme P. 2018; Prevention and treatment of chronic postsurgical pain: a narrative review. Drugs. 78:339–54. DOI: 10.1007/s40265-018-0866-x. PMID: 29380289.
41. Buvanendran A, Della Valle CJ, Kroin JS, Shah M, Moric M, Tuman KJ, et al. 2019; Acute postoperative pain is an independent predictor of chronic postsurgical pain following total knee arthroplasty at 6 months: a prospective cohort study. Reg Anesth Pain Med. 44:e100036. DOI: 10.1136/rapm-2018-100036. PMID: 30770420.
42. Kehlet H, Jensen TS, Woolf CJ. 2006; Persistent postsurgical pain: risk factors and prevention. Lancet. 367:1618–25. DOI: 10.1016/S0140-6736(06)68700-X. PMID: 16698416.
43. Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. 2006; Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 7:626–34. DOI: 10.1016/j.jpain.2006.02.007. PMID: 16942948. PMCID: PMC6983301.
44. Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. 2006; Chronic pain following total hip arthroplasty: a nationwide questionnaire study. Acta Anaesthesiol Scand. 50:495–500. DOI: 10.1111/j.1399-6576.2006.00976.x. PMID: 16548863.
45. L'Hermite J, Pagé MG, Chevallier T, Occean B, Viel E, Bredeau O, et al. 2021; Characterisation of pragmatic postoperative PAin Trajectories over seven days and their association with CHronicity after 3 months: a prospective, pilot cohort study (PATCH study). Anaesth Crit Care Pain Med. 40:100793. DOI: 10.1016/j.accpm.2020.100793. PMID: 33359373.
46. Imai R, Nishigami T, Kubo T, Ishigaki T, Yonemoto Y, Mibu A, et al. 2021; Using a postoperative pain trajectory to predict pain at 1 year after total knee arthroplasty. Knee. 32:194–200. DOI: 10.1016/j.knee.2021.08.021. PMID: 34509825.
47. Willingham M, Rangrass G, Curcuru C, Ben Abdallah A, Wildes TS, McKinnon S, et al. 2020; Association between postoperative complications and lingering post-surgical pain: an observational cohort study. Br J Anaesth. 124:214–21. DOI: 10.1016/j.bja.2019.10.012. PMID: 31771788.
48. Yoon S, Hong WP, Joo H, Jang D, Park S, Lee HJ. 2021; Adjuvant chemotherapy as a risk factor for chronic postoperative pain after video-assisted thoracoscopic surgery: a 10-year single-centre retrospective study. Interact Cardiovasc Thorac Surg. 32:276–83. DOI: 10.1093/icvts/ivaa250. PMID: 33236038. PMCID: PMC8906664.
49. Papadomanolakis-Pakis N, Uhrbrand P, Haroutounian S, Nikolajsen L. 2021; Prognostic prediction models for chronic postsurgical pain in adults: a systematic review. Pain. 162:2644–57. DOI: 10.1097/j.pain.0000000000002261. PMID: 34652320.
50. Montes A, Roca G, Cantillo J, Sabate S. 2020; Presurgical risk model for chronic postsurgical pain based on 6 clinical predictors: a prospective external validation. Pain. 161:2611–8. DOI: 10.1097/j.pain.0000000000001945. PMID: 32541391.
51. Meretoja TJ, Andersen KG, Bruce J, Haasio L, Sipilä R, Scott NW, et al. 2017; Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 35:1660–7. DOI: 10.1200/JCO.2016.70.3413. PMID: 28524782.
52. van Driel MEC, van Dijk JFM, Baart SJ, Meissner W, Huygen FJPM, Rijsdijk M. 2022; Development and validation of a multivariable prediction model for early prediction of chronic postsurgical pain in adults: a prospective cohort study. Br J Anaesth. 129:407–15. DOI: 10.1016/j.bja.2022.04.030. PMID: 35732539.
53. Chapman CR, Vierck CJ. 2017; The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 18:359.e1–38. DOI: 10.1016/j.jpain.2016.11.004. PMID: 27908839.
54. Hankerd K, McDonough KE, Wang J, Tang SJ, Chung JM, La JH. 2022; Postinjury stimulation triggers a transition to nociplastic pain in mice. Pain. 163:461–73. DOI: 10.1097/j.pain.0000000000002366. PMID: 34285154. PMCID: PMC8669020.
55. Xuan C, Yan W, Wang D, Li C, Ma H, Mueller A, et al. 2022; Efficacy of preemptive analgesia treatments for the management of postoperative pain: a network meta-analysis. Br J Anaesth. 129:946–58. DOI: 10.1016/j.bja.2022.08.038. PMID: 36404458.
56. Woolf CJ, Chong MS. 1993; Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 77:362–79. DOI: 10.1213/00000539-199377020-00026. PMID: 8346839.
57. Şentürk M, Özcan PE, Talu GK, Kiyan E, Çamci E, Özyalçin S, et al. 2002; The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 94:11–5. DOI: 10.1213/00000539-200201000-00003. PMID: 11772793.
58. Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. 2006; Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 103:703–8. DOI: 10.1213/01.ane.0000230603.92574.4e. PMID: 16931684.
59. Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, et al. 2011; Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology. 114:1144–54. DOI: 10.1097/ALN.0b013e31820fc7d2. PMID: 21368651.
60. Park SK, Yoon S, Kim BR, Choe SH, Bahk JH, Seo JH. 2020; Pre-emptive epidural analgesia for acute and chronic post-thoracotomy pain in adults: a systematic review and meta-analysis. Reg Anesth Pain Med. 45:1006–16. DOI: 10.1136/rapm-2020-101708. PMID: 33055105.
61. Irwin MG, Chung CKE, Ip KY, Wiles MD. 2020; Influence of propofol-based total intravenous anaesthesia on peri-operative outcome measures: a narrative review. Anaesthesia. 75 Suppl 1:e90–100. DOI: 10.1111/anae.14905.
62. Ogurlu M, Sari S, Küçük M, Bakiş M, Uğur B, Eshraghi YE, et al. 2014; Comparison of the effect of propofol and sevoflurane anaesthesia on acute and chronic postoperative pain after hysterectomy. Anaesth Intensive Care. 42:365–70. DOI: 10.1177/0310057X1404200314. PMID: 24794477.
63. Chan MTV, Wan ACM, Gin T, Leslie K, Myles PS. 2011; Chronic postsurgical pain after nitrous oxide anesthesia. Pain. 152:2514–20. DOI: 10.1016/j.pain.2011.07.015. PMID: 21889262.
64. Chan MT, Peyton PJ, Myles PS, Leslie K, Buckley N, Kasza J, et al. 2016; Chronic postsurgical pain in the Evaluation of Nitrous Oxide in the Gas Mixture for Anaesthesia (ENIGMA)-II trial. Br J Anaesth. 117:801–11. Erratum in: Br J Anaesth 2017; 119: 851. DOI: 10.1093/bja/aew338. PMID: 27956679.
65. de Hoogd S, Ahlers SJ, van Dongen EP, van de Garde EM, Hamilton-Ter Brake TA, Dahan A, et al. 2016; Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. Clin J Pain. 32:726–35. DOI: 10.1097/AJP.0000000000000317. PMID: 26626296.
66. Meijer FS, Niesters M, van Velzen M, Martini CH, Olofsen E, Edry R, et al. 2020; Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials. J Clin Monit Comput. 34:629–41. DOI: 10.1007/s10877-019-00362-4. PMID: 31327102. PMCID: PMC7367908.
67. Rivat C, Bollag L, Richebé P. 2013; Mechanisms of regional anaesthesia protection against hyperalgesia and pain chronicization. Curr Opin Anaesthesiol. 26:621–5. DOI: 10.1097/01.aco.0000432511.08070.de. PMID: 23995064.
68. Andreae MH, Andreae DA. 2013; Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 111:711–20. DOI: 10.1093/bja/aet213. PMID: 23811426. PMCID: PMC3793661.
69. Levene JL, Weinstein EJ, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. 2019; Local anesthetics and regional anesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children: a Cochrane systematic review and meta-analysis update. J Clin Anesth. 55:116–27. DOI: 10.1016/j.jclinane.2018.12.043. PMID: 30640059. PMCID: PMC6461051.
70. Na HS, Koo CH, Koo BW, Ryu JH, Jo H, Shin HJ. 2023; Effect of the paravertebral block on chronic postsurgical pain after thoracic surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 37:252–60. DOI: 10.1053/j.jvca.2022.10.029. PMID: 36428202.
71. Albi-Feldzer A, Mouret-Fourme E E, Hamouda S, Motamed C, Dubois PY, Jouanneau L, et al. 2013; A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: effects on chronic postoperative pain. Anesthesiology. 118:318–26. DOI: 10.1097/ALN.0b013e31827d88d8. PMID: 23340351.
72. Harkouk H, Fletcher D, Martinez V. 2021; Paravertebral block for the prevention of chronic postsurgical pain after breast cancer surgery. Reg Anesth Pain Med. 46:251–7. DOI: 10.1136/rapm-2020-102040. PMID: 33414157.
73. Borghi B, D'Addabbo M, White PF, Gallerani P, Toccaceli L, Raffaeli W, et al. 2010; The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth Analg. 111:1308–15. DOI: 10.1213/ANE.0b013e3181f4e848. PMID: 20881281.
74. Qian B, Huang S, Liao X, Wu J, Lin Q, Lin Y. 2021; Serratus anterior plane block reduces the prevalence of chronic postsurgical pain after modified radical mastectomy: a randomized controlled trial. J Clin Anesth. 74:110410. DOI: 10.1016/j.jclinane.2021.110410. PMID: 34175638.
75. Covotta M, Claroni C, Costantini M, Torregiani G, Pelagalli L, Zinilli A, et al. 2020; The effects of ultrasound-guided transversus abdominis plane block on acute and chronic postsurgical pain after robotic partial nephrectomy: a prospective randomized clinical trial. Pain Med. 21:378–86. DOI: 10.1093/pm/pnz214. PMID: 31504875.
76. Wiech M, Żurek S, Kurowicki A, Horeczy B, Czuczwar M, Piwowarczyk P, et al. 2022; Erector spinae plane block decreases chronic postoperative pain severity in patients undergoing coronary artery bypass grafting. J Clin Med. 11:5949. DOI: 10.3390/jcm11195949. PMID: 36233819. PMCID: PMC9571025. PMID: f496a77a6f9045b58a06e62b98c8d1e1.
77. Geradon P, Lavand'homme P. 2022; Use of regional analgesia to prevent the conversion from acute to chronic pain. Curr Opin Anaesthesiol. 35:641–6. DOI: 10.1097/ACO.0000000000001175. PMID: 35942702.
78. Pogatzki-Zahn EM, Segelcke D, Schug SA. 2017; Postoperative pain-from mechanisms to treatment. Pain Rep. 2:e588. DOI: 10.1097/PR9.0000000000000588. PMID: 29392204. PMCID: PMC5770176. PMID: ac8c3fa6919b44b28925d844cc9b338d.
79. Nielsen RV, Fomsgaard JS, Nikolajsen L, Dahl JB, Mathiesen O. 2019; Intraoperative S-ketamine for the reduction of opioid consumption and pain one year after spine surgery: a randomized clinical trial of opioid-dependent patients. Eur J Pain. 23:455–60. DOI: 10.1002/ejp.1317. PMID: 30246357.
80. Brinck ECV, Maisniemi K, Kankare J, Tielinen L, Tarkkila P, Kontinen VK. 2021; Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg. 132:69–79. DOI: 10.1213/ANE.0000000000004729. PMID: 32167978.
81. Schug SA, Peyton P. 2017; Does perioperative ketamine have a role in the prevention of chronic postsurgical pain: the ROCKet trial. Br J Pain. 11:166–8. DOI: 10.1177/2049463717736076. PMID: 29123660. PMCID: PMC5661694.
82. Carley ME, Chaparro LE, Choinière M, Kehlet H, Moore RA, Van Den Kerkhof E, et al. 2021; Pharmacotherapy for the prevention of chronic pain after surgery in adults: an updated systematic review and meta-analysis. Anesthesiology. 135:304–25. DOI: 10.1097/ALN.0000000000003837. PMID: 34237128.
83. Horn A, Kaneshiro K, Tsui BCH. 2020; Preemptive and preventive pain psychoeducation and its potential application as a multimodal perioperative pain control option: a systematic review. Anesth Analg. 130:559–73. DOI: 10.1213/ANE.0000000000004319. PMID: 31335400.
84. Wang L, Chang Y, Kennedy SA, Hong PJ, Chow N, Couban RJ, et al. 2018; Perioperative psychotherapy for persistent post-surgical pain and physical impairment: a meta-analysis of randomised trials. Br J Anaesth. 120:1304–14. DOI: 10.1016/j.bja.2017.10.026. PMID: 29793597.
85. Katz J, Weinrib A, Fashler SR, Katznelzon R, Shah BR, Ladak SS, et al. 2015; The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 8:695–702. DOI: 10.2147/JPR.S91924. PMID: 26508886. PMCID: PMC4610888.
86. Flatters SJ. 2008; Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain. 135:119–30. DOI: 10.1016/j.pain.2007.05.013. PMID: 17590272. PMCID: PMC2278124.
87. Landreneau RJ, Mack MJ, Hazelrigg SR, Naunheim K, Dowling RD, Ritter P, et al. 1994; Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 107:1079–85. DOI: 10.1016/S0022-5223(94)70384-1. PMID: 8159030.
88. Wildgaard K, Ravn J, Nikolajsen L, Jakobsen E, Jensen TS, Kehlet H. 2011; Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand. 55:60–8. DOI: 10.1111/j.1399-6576.2010.02357.x. PMID: 21077845.
89. Fregoso G, Wang A, Tseng K, Wang J. 2019; Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician. 22:479–88. DOI: 10.36076/ppj/2019.22.479. PMID: 31561647.
90. Jin J, Du X, Min S, Liu L. 2022; Comparison of chronic postsurgical pain between single-port and multi-port video-assisted thoracoscopic pulmonary resection: a prospective study. Thorac Cardiovasc Surg. 70:430–8. DOI: 10.1055/s-0042-1744546. PMID: 35439833. PMCID: PMC9365528.
91. Yang J, Bauer BA, Wahner-Roedler DL, Chon TY, Xiao L. 2020; The modified WHO analgesic ladder: is it appropriate for chronic non-cancer pain? J Pain Res. 13:411–7. DOI: 10.2147/JPR.S244173. PMID: 32110089. PMCID: PMC7038776.
92. Kim EJ, Hwang EJ, Yoo YM, Kim KH. 2022; Prevention, diagnosis, and treatment of opioid use disorder under the supervision of opioid stewardship programs: it's time to act now. Korean J Pain. 35:361–82. DOI: 10.3344/kjp.2022.35.4.361. PMID: 36175336. PMCID: PMC9530691.
93. Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. 2013; The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 154:95–102. DOI: 10.1016/j.pain.2012.09.010. PMID: 23273105.
94. Dualé C, Ouchchane L, Schoeffler P, Dubray C. 2014; Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain. 15:24.e1–20. DOI: 10.1016/j.jpain.2013.08.014. PMID: 24373573.
95. Thapa P, Euasobhon P. 2018; Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 31:155–73. DOI: 10.3344/kjp.2018.31.3.155. PMID: 30013730. PMCID: PMC6037807.
96. Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. 2019; A comprehensive algorithm for management of neuropathic pain. Pain Med. 20(Suppl 1):S2–12. Erratum in: Pain Med 2023; 24: 219. DOI: 10.1093/pm/pnz075. PMID: 31152178. PMCID: PMC6544553.
97. Wylde V, Dennis J, Beswick AD, Bruce J, Eccleston C, Howells N, et al. 2017; Systematic review of management of chronic pain after surgery. Br J Surg. 104:1293–306. DOI: 10.1002/bjs.10601. PMID: 28681962. PMCID: PMC5599964.
98. Nicholls JL, Azam MA, Burns LC, Englesakis M, Sutherland AM, Weinrib AZ, et al. 2018; Psychological treatments for the management of postsurgical pain: a systematic review of randomized controlled trials. Patient Relat Outcome Meas. 9:49–64. DOI: 10.2147/PROM.S121251. PMID: 29403322. PMCID: PMC5783145.
99. Albayrak I, Apiliogullari S, Dal CN, Levendoglu F, Ozerbil OM. 2017; Efficacy of pulsed radiofrequency therapy to dorsal root ganglion adding to TENS and exercise for persistent pain after total knee arthroplasty. J Knee Surg. 30:134–42. DOI: 10.1055/s-0036-1583268. PMID: 27123667.
100. Kim DJ, Shen S, Hanna GM. 2017; Ultrasound-guided radiofrequency lesioning of the articular branches of the femoral nerve for the treatment of chronic post-arthroplasty hip pain. Pain Physician. 20:E323–7. DOI: 10.36076/ppj.2017.E327. PMID: 28158168.
101. Hetta DF, Mahran AM, Kamal EE. 2018; Pulsed radiofrequency treatment for chronic post-surgical orchialgia: a double-blind, sham-controlled, randomized trial: three-month results. Pain Physician. 21:199–205. DOI: 10.36076/ppj.2018.2.199. PMID: 29565950.
102. Abbas DN, Reyad RM. 2018; Thermal versus super voltage pulsed radiofrequency of stellate ganglion in post-mastectomy neuropathic pain syndrome: a prospective randomized trial. Pain Physician. 21:351–62. DOI: 10.36076/ppj.2018.4.351. PMID: 30045592.
103. Antony AB, Schultheis BC, Jolly SM, Bates D, Hunter CW, Levy RM. 2019; Neuromodulation of the dorsal root ganglion for chronic postsurgical pain. Pain Med. 20(Suppl 1):S41–6. DOI: 10.1093/pm/pnz072. PMID: 31152174. PMCID: PMC6733040.
104. Gupta M, Scowcroft J, Kloster D, Guirguis M, Carlson J, McJunkin T, et al. 2020; 10-kHz spinal cord stimulation for chronic postsurgical pain: results from a 12-month prospective, multicenter study. Pain Pract. 20:908–18. DOI: 10.1111/papr.12929. PMID: 32585742. PMCID: PMC7754504.
105. Lo Bianco G, Papa A, Gazzerro G, Rispoli M, Tammaro D, Di Dato MT, et al. 2021; Dorsal root ganglion stimulation for chronic postoperative pain following thoracic surgery: a pilot study. Neuromodulation. 24:774–8. DOI: 10.1111/ner.13265. PMID: 32909359.
106. Cohen MA, Emerick T. 2022; Understanding the role of pulsed radiofrequency in the early management of chronic postsurgical groin pain. Pain Med. 23:1186–98. DOI: 10.1093/pm/pnab324. PMID: 34791420.
107. Abid Azam M, Weinrib AZ, Montbriand J, Burns LC, McMillan K, Clarke H, et al. 2017; Acceptance and Commitment Therapy to manage pain and opioid use after major surgery: preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain. 1:37–49. DOI: 10.1080/24740527.2017.1325317. PMID: 35005340. PMCID: PMC8730651.
108. Katz J, Weinrib AZ, Clarke H. 2019; Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 3:49–58. DOI: 10.1080/24740527.2019.1574537. PMID: 35005419. PMCID: PMC8730596. PMID: bf98a3f1ebe04ec7b69093906f6b35f8.
109. Buys MJ, Bayless K, Romesser J, Anderson Z, Patel S, Zhang C, et al. 2020; Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 45:847–52. DOI: 10.1136/rapm-2020-101797. PMID: 32848086. PMCID: PMC7784497.
110. Bleicher J, Brooke BS, Bayless K, Anderson Z, Beckstrom J, Zhang C, et al. 2022; Postoperative opioid prescribing, use and pain trends following general surgery procedures: a retrospective cohort study among veterans comparing non-opioid versus chronic opioid users. Reg Anesth Pain Med. 47:487–93. DOI: 10.1136/rapm-2021-103382. PMID: 35523480.
111. Featherall J, Anderson JT, Anderson LA, Bayless K, Anderson Z, Brooke BS, et al. 2022; A multidisciplinary transitional pain management program is associated with reduced opioid dependence after primary total joint arthroplasty. J Arthroplasty. 37:1048–53. DOI: 10.1016/j.arth.2022.02.032. PMID: 35181448.
112. Yu HC, Kleiman V, Kojic K, Slepian PM, Cortes H, McRae K, et al. 2022; Prevention and management of chronic postsurgical pain and persistent opioid use following solid organ transplantation: experiences from the Toronto General Hospital Transitional Pain Service. Transplantation. doi: 10.1097/TP.0000000000004441. DOI: 10.1097/TP.0000000000004441. PMID: 36482750.
113. Sun EC, Mariano ER, Narouze S, Gabriel RA, Elsharkawy H, Gulur P, et al. 2021; Making a business plan for starting a transitional pain service within the US healthcare system. Reg Anesth Pain Med. 46:727–31. DOI: 10.1136/rapm-2021-102669. PMID: 33879540.
Fig. 1
Schematic diagram of interventions for the management of chronic postsurgical pain (CPSP) by perioperative time flow. Routine management for all surgical patients is presented in blue colored rectangles, conditions requiring additional interventions are presented in yellow colored pentagons, and further treatment options are presented in green colored rectangles.
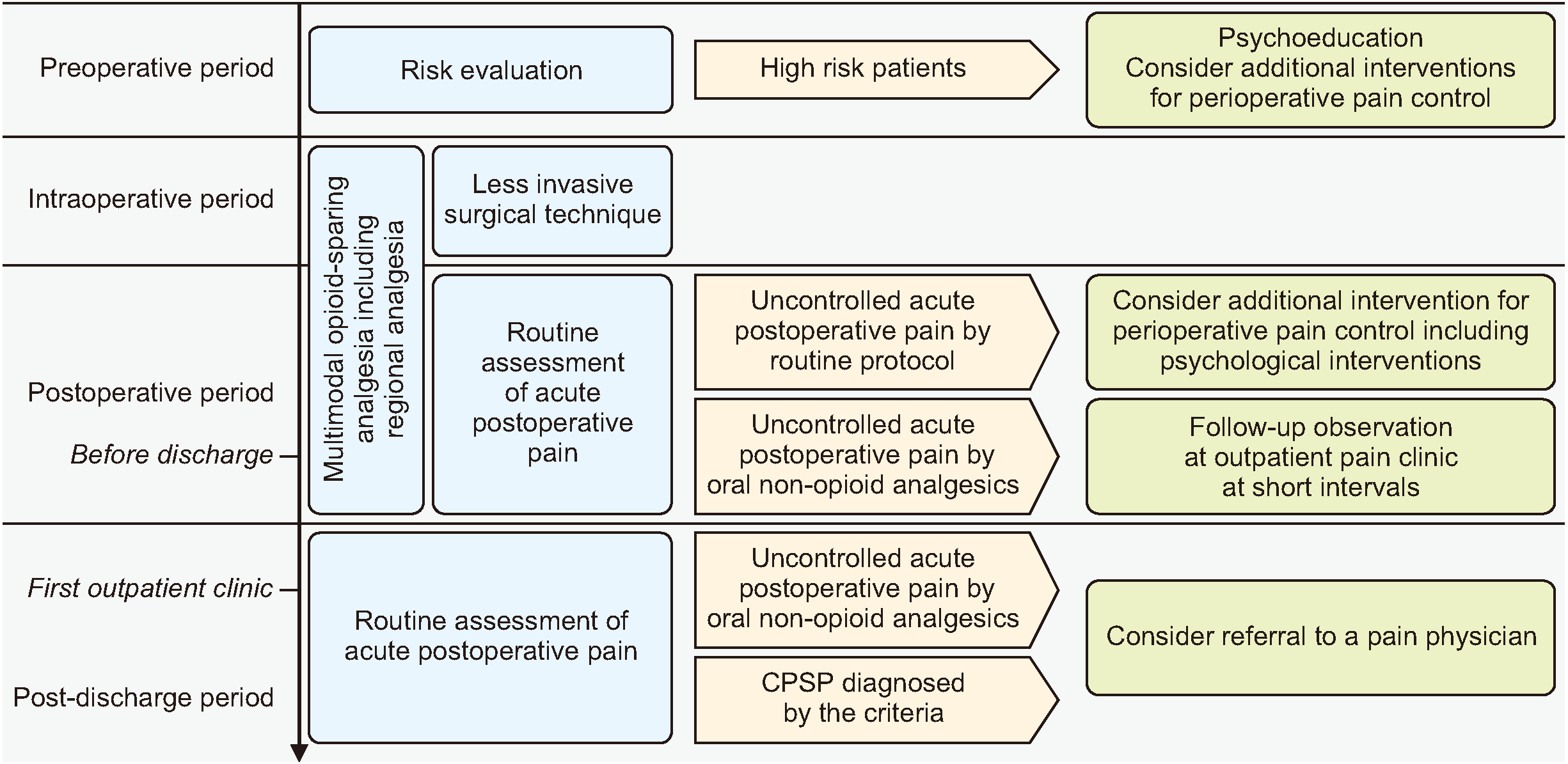
Table 1
Contribution of previous literature to the latest definition of chronic postsurgical pain in the ICD-11
| Literature | Macrae [12] | Werner and Kongsgaard [13] | Treede et al. [14] | Schug et al. [15] |
|---|---|---|---|---|
| Temporal relationship with surgery | The pain develops after the surgery | The pain develops or increases in intensity after the surgery | The pain develops after the surgery | The pain develops or increases in intensity after the surgery |
| Duration of pain after surgery | At least 2 months’ duration | At least 3–6 months’ duration | At least 3 months’ duration | |
| Exclusion of other causes | Other causes of pain need to be excluded; pain continuing from a pre-existing pain-related condition should also be excluded | Other causes of pain need to be excluded | Other causes of pain need to be excluded; pain continuing from a pre-existing pain-related condition should also be excluded | |
| Location of pain | - | The pain is either localized to the surgical field, projected to the innervation territory of a nerve situated in this area, or referred to a dermatome | - | The pain is either localized to the surgical field, projected to the innervation territory of a nerve situated in this area, or referred to a dermatome |
| Others | - | The pain is either a continuation of acute postsurgical pain or develops after an asymptomatic period | CPSP is often neuropathic | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download