Abstract
Neuroendocrine carcinoma (NEC) arising from the extrahepatic bile duct is extremely rare and commonly mistaken for cholangiocarcinoma. Therefore, NEC of the bile duct is difficult to diagnose preoperatively. Previously reported cases were resected with a diagnosis of cholangiocarcinoma and diagnosed with NEC after surgery. This paper reports an 84-year-old female with small-cell NEC of the extrahepatic bile duct, confirmed by a biopsy from an ERCP, with a review of the relevant literature. Contrast-enhanced abdomen computed tomography and magnetic resonance cholangiopancreatography revealed an approximately 1.7 cm enhancing intraductal mass in the proximal common bile duct with dilatation of the upstream bile duct. ERCP showed a long strictured segment in the proximal common bile duct with bile duct dilatation. A biopsy was performed at the site of the stricture. Histological examinations and hematoxylin–eosin staining showed the solid proliferation of small tumor cells with irregularly shaped hyperchromatic nuclei. Immunohistochemical examinations showed that the tumor cells were positive for CD56 and synaptophysin. Small-cell NEC of the extrahepatic bile duct was confirmed based on the histology and immunohistochemistry findings. The patient and their family denied treatment because of the patient’s old age.
Neuroendocrine carcinoma (NEC) arising from the bile duct is an extremely rare neoplasm commonly mistaken for cholangiocarcinoma. Therefore, NEC of the bile duct is difficult to diagnose preoperatively. Most reported cases were resected with a diagnosis of cholangiocarcinoma and diagnosed with NEC after surgery.1-5
This paper reports the case of an 84-year-old female with small-cell NEC of the extrahepatic bile duct, confirmed by a biopsy from an ERCP, with a review of the relevant literature on this condition.
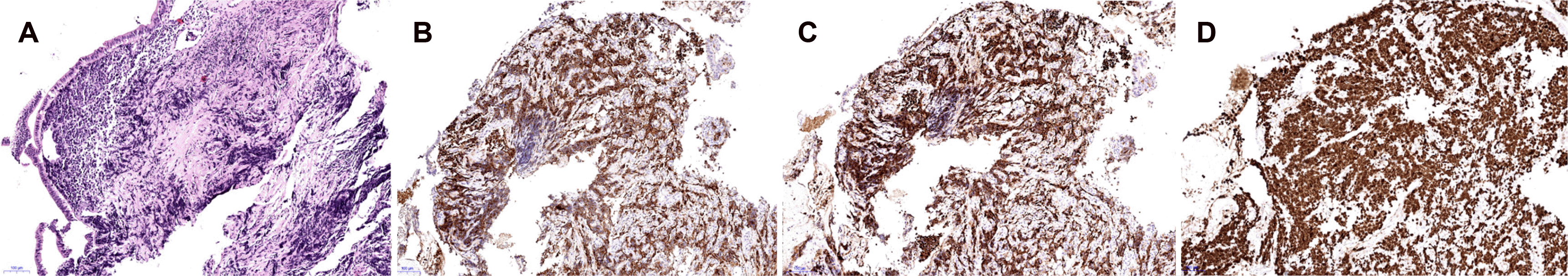
An 84-year-old female was admitted to hospital with a history of right upper quadrant pain for the past two months. The pain did not radiate along the back. The patient complained of anorexia and nausea. The patient had a prior medical history of diabetes mellitus, hypertension, and dyslipidemia. The patient had no history of drinking alcohol or smoking. Upon admission, the patient was afebrile, and their blood pressure and pulse were normal. Scleral icterus was present. The abdomen was mildly distended, with tenderness in the right upper quadrant. There were no abdominal masses, and the liver, gallbladder, and spleen were not palpable. Laboratory examinations revealed the following findings: white blood cell count 8,700 /mm3 (normal range: 6,000–10,000 /mm3), hemoglobin 12.4 g/dL (normal range: 12–16 g/dL), platelet count 149,000 /mm3 (normal range: 130,000–450,000 /mm3), blood urea nitrogen 22.9 mg/dL (normal range: 8–23 mg/dL), creatinine 1.07 mg/dL (normal range: 0.5–1.3 mg/dL), serum albumin 3.0 g/dL (normal range: 3.0–5.0 g/dL), aspartate aminotransferase 148 U/L (normal range: 5–37 U/L), alanine aminotransferase 208 U/L (normal range: 5–40 U/L), alkaline phosphatase 511 U/L (normal range: 39–117 U/L), γ-glutamyl transpeptidase 1201 U/L (normal range: 7–49 U/L), total bilirubin 9.11 mg/dL with a direct fraction of 8.57 mg/dL (normal range: 0.2–1.2 mg/dL and 0.05–0.3 mg/dL), and c-reactive protein level 14.6 mg/L (normal range: 0–0.3). Amylase and lipase were within the normal limits. The serum CEA level was 1.75 ng/mL (normal range: 0–5 ng/mL). The CA 19-9 level was 12,000 U/mL (normal range: 0–39 u/mL). A contrast-enhanced abdomen and pelvis CT revealed an approximately 1.7 cm enhancing intraductal mass in the proximal common bile duct with dilatation of the upstream bile duct and a 3.1 cm irregular enhancing mass along the common hepatic chain suggesting metastatic lymph node (Fig. 1A, B). MRCP revealed an intraductal mass in the proximal common bile duct (Fig. 1C). The patient was initially diagnosed with cholangiocarcinoma based on the imaging findings. ERCP revealed a long strictured segment in the proximal portion of the common bile duct with bile duct dilatation (Fig. 2A). A biopsy was performed at the site of the biliary stricture, and biliary drainage with a plastic stent (7 Fr, 7 cm) was done (Fig. 2B). Routine histology stained with hematoxylin–eosin showed the solid and cord-structured proliferation of small tumor cells with irregularly shaped hyperchromatic nuclei and scanty cytoplasm (Fig. 3A). An immunohistochemical examination was performed to clarify the origin of the tumor cells. The tumor cells were positive for CD56 and synaptophysin (Fig. 3B, C). In addition, the Ki-67 labeling index was 85% (Fig. 3D). Small-cell NEC of the extrahepatic bile duct was confirmed based on the histology and immunohistochemistry findings. The national comprehensive cancer network guidelines,8 the biopsy specimens with CT and MRCP findings were interpreted comprehensively as stage III (cT3N1M0) small-cell type NEC according to the World Health Organization (WHO) classification and grading criteria for neuroendocrine neoplasms of the gastrointestinal tract and hepatopancreatic biliary organs.6,7 The patient and their family denied treatments because of their old age. Informed consent was obtained from the patient for publication.
Neuroendocrine neoplasms (NENs) derived from neuroendocrine cells usually develop in the digestive and respiratory systems. NENs arising from the biliary system and gallbladder are rare and represent 0.2–2% of all NENs.6-8 NENs have a range of clinical outcomes, including indolent and aggressive features depending on the differentiation and proliferation grading.6-8 NENs are classified as well-differentiated neuroendocrine tumors (NETs), poorly differentiated small-cell and large-cell NECs, and mixed neuroendocrine-non-neuroendocrine neoplasms according to the WHO classification and grading criteria of neuroendocrine neoplasms. NETs are graded as G1, G2, and G3 based on proliferation measurements such as mitotic rate and Ki-67 index. High-grade NECs are defined by a mitotic rate over 20 mitoses/2 mm2, and a Ki-67 index of more than 20%.6-8
NECs arising from the extrahepatic bile duct are extremely rare. NECs of the extrahepatic bile duct are commonly mistaken for cholangiocarcinomas owing to their rarity. NECs are very difficult to diagnose preoperatively. Most of the reported cases have been diagnosed retrospectively after surgery by pathology and immunohistochemical staining, including synaptophysin, CD56, and chromogranin.1-5
In the cases reported in the literature,9,10 the median age of patients with NEC of the extrahepatic bile duct was 70.0 years old. The sex predisposition was predominantly male. The most common clinical presentation was often jaundice, followed by abdominal pain, weight loss, and nausea. The most common site was the distal common bile duct, followed by the perihilar bile duct. The diagnostic rates through bile duct biopsies and brush cytology are very low. Only a few cases were diagnosed as NEC before surgery.11-13 NECs are subclassified into small-cell and large-cell NECs with poor differentiation.6-8 Most NECs of the extrahepatic bile duct cases were small-cell NECs.14-18
In this case, an NEC of the extrahepatic bile duct manifested in an 84-year-old female with jaundice and abdominal pain. The clinical and radiologic imaging diagnosis strongly suggested cholangiocarcinoma. On the other hand, a biopsy from the ERCP showed small-cell NEC with positivity for immunohistochemical staining of synaptophysin and CD56 and 85% Ki-67 labeling index.
The treatment of this disease is not well established. The common surgical procedure is pancreatoduodenectomy, followed by bile duct resection and hepatectomy with bile duct resection according to the tumor location.9,10 Most reported cases were clinically aggressive and led to a poor prognosis, even in patients with clinically localized and surgical complete resections. In a review of 22 patients with biliary NEC, distant metastasis was found in 16 cases, and all patients showed distant metastasis within one year after surgery. The median overall survival was only 12 months (95% confidence interval, 5–20 months) in the 21 cases with follow-up data.10 The most common recurrent organ was the liver, followed by lymph nodes, lungs, and bones.9 The effectiveness of adjuvant and neoadjuvant chemotherapies is still difficult to assess because there are few treatment cases.10-13,19,20
In summary, NECs of the extrahepatic bile duct are extremely rare and difficult to diagnose preoperatively. In addition, this disease can progress rapidly after surgical resection and has a poor prognosis. Therefore, in the future, aggressive multidisciplinary treatments, including surgery, chemotherapy, and radiotherapy, are needed to improve the prognosis of this disease.
REFERENCES
1. Luchini C, Pelosi G, Scarpa A, et al. 2021; Neuroendocrine neoplasms of the biliary tree, liver and pancreas: a pathological approach. Pathologica. 113:28–38. DOI: 10.32074/1591-951X-231. PMID: 33686308. PMCID: PMC8138696.
2. Hong N, Kim HJ, Byun JH, et al. 2015; Neuroendocrine neoplasms of the extrahepatic bile duct: radiologic and clinical characteristics. Abdom Imaging. 40:181–191. DOI: 10.1007/s00261-014-0191-0. PMID: 25008023.
3. Michalopoulos N, Papavramidis TS, Karayannopoulou G, Pliakos I, Papavramidis ST, Kanellos I. 2014; Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res. 20:765–775. DOI: 10.1007/s12253-014-9808-4. PMID: 24917351.
4. Ishida M, Okano K, Sandoh K, et al. 2020; Neuroendocrine carcinoma diagnosis from bile duct cytological specimens: A retrospective single-center study. Diagn Cytopathol. 48:154–158. DOI: 10.1002/dc.24334. PMID: 31697402.
5. Zheng Z, Chen C, Li B, et al. 2019; Biliary neuroendocrine neoplasms: clinical profiles, management, and analysis of prognostic factors. Front Oncol. 9:38. DOI: 10.3389/fonc.2019.00038. PMID: 30805307. PMCID: PMC6370735.
6. Nagtegaal ID, Odze RD, Klimstra D, et al. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76:182–188. DOI: 10.1111/his.13975. PMID: 31433515. PMCID: PMC7003895.
7. Rindi G, Mete O, Uccella S, et al. 2022; Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 33:115–154. DOI: 10.1007/s12022-022-09708-2. PMID: 35294740.
8. Fernandes CJ, Leung G, Eads JR, Katona BW. 2022; Gastroenteropancreatic neuroendocrine tumors. Gastroenterol Clin North Am. 51:625–647. DOI: 10.1016/j.gtc.2022.06.002. PMID: 36153114.
9. Shim JR, Kim JR, Park Y, Seo HI. 2020; Small-cell neuroendocrine carcinoma arising from an extra-hepatic bile duct: a case report. Gastroenterol Rep (Oxf). 9:380–382. DOI: 10.1093/gastro/goaa051. PMID: 34567573. PMCID: PMC8460092.
10. Kamiya M, Yamamoto N, Kamioka Y, et al. 2020; Rapidly progressed neuroendocrine carcinoma in the extrahepatic bile duct: a case report and review of the literature. Surg Case Rep. 6:191. DOI: 10.1186/s40792-020-00945-3. PMID: 32748005. PMCID: PMC7399003.
11. Umezaki N, Hashimoto D, Yamashita YI, et al. 2019; Neuroendocrine tumor of the hilar bile duct. Anticancer Res. 39:903–907. DOI: 10.21873/anticanres.13192. PMID: 30711974.
12. Hazama K, Suzuki Y, Takahashi M, et al. 2003; Primary small cell carcinoma of the common bile duct, in which surgical treatment was performed after neoadjuvant chemotherapy: report of a case. Surg Today. 33:870–872. DOI: 10.1007/s00595-003-2594-3. PMID: 14605962.
13. Okamura Y, Maeda A, Matsunaga K, et al. 2009; Small-cell carcinoma in the common bile duct treated with multidisciplinary management. J Hepatobiliary Pancreat Surg. 16:575–578. DOI: 10.1007/s00534-009-0051-4. PMID: 19288048.
14. Aigner B, Kornprat P, Schöllnast H, Kasparek AK, Mischinger HJ, Haybaeck J. 2015; A case of focal small-cell neuroendocrine carcinoma in the vicinity of the extrahepatic bile duct, adjacent to an extensive biliary intraepithelial neoplasm: a diagnostic challenge with major clinical implications. Anticancer Res. 35:4821–4828.
15. Jeon WJ, Chae HB, Park SM, Youn SJ, Choi JW, Kim SH. 2006; [A case of primary small cell carcinoma arising from the common bile duct]. Korean J Gastroenterol. 48:438–442. Korean.
16. Kwon YD, Kim CD, Kim YS, et al. 2009; A case of synchronous primary cancer: small cell carcinoma in the common bile duct and adenocarcinoma in the stomach. Korean J Gastrointest Endosc. 38:303–308.
17. Kim BC, Song TJ, Lee H, et al. 2013; [A case of small cell neuroendocrine tumor occurring at hilar bile duct]. Korean J Gastroenterol. 62:301–305. Korean. DOI: 10.4166/kjg.2013.62.5.301. PMID: 24262597.
18. Sugita H, Maeda K, Nishikawa S, Doden K, Hashizume Y. 2022; Case of resected small-cell neuroendocrine carcinoma of the extrahepatic bile duct. J Surg Case Rep. 2022:rjac020. DOI: 10.1093/jscr/rjac020. PMID: 35154639. PMCID: PMC8828790.
19. Garcia-Carbonero R, Sorbye H, Baudin E, et al. Vienna Consensus Conference participants. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016; 103:186–194. DOI: 10.1159/000443172. PMID: 26731334.
20. Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. 2014; Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 120:2814–2823. DOI: 10.1002/cncr.28721. PMID: 24771552.
Fig. 1
(A, B) Contrast-enhanced abdomen and pelvis CT revealed an approximately 1.7 cm enhancing intraductal mass in the proximal common bile duct with dilatation of the upstream bile duct (square), and a 3.1 cm irregular enhancing mass along common hepatic chain suggesting metastatic lymph node (circle). (C) MRCP revealed an intraductal mass in the proximal common bile duct.
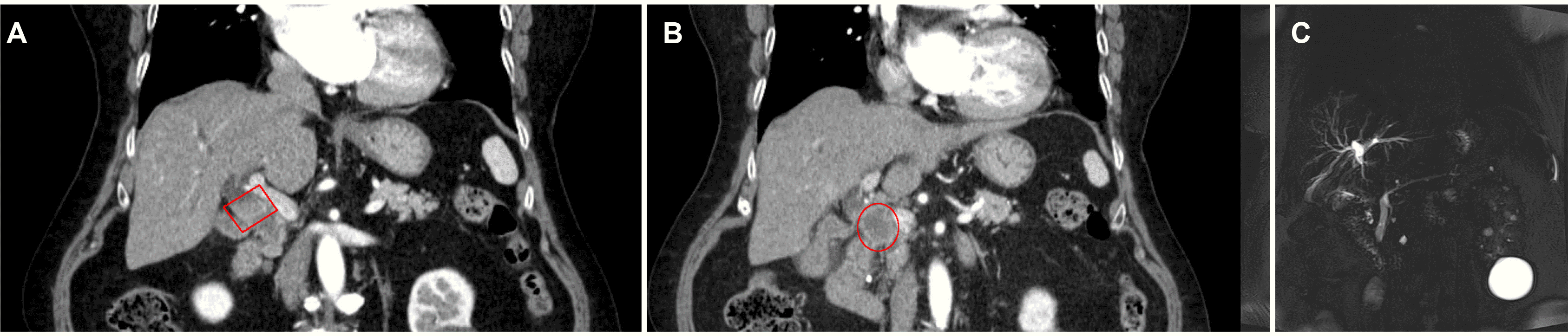
Fig. 2
(A) ERCP revealed a long strictured segment in the proximal portion of the common bile duct with bile duct dilatation. (B) Biliary drainage was performed with a plastic stent (7 Fr, 7 cm).
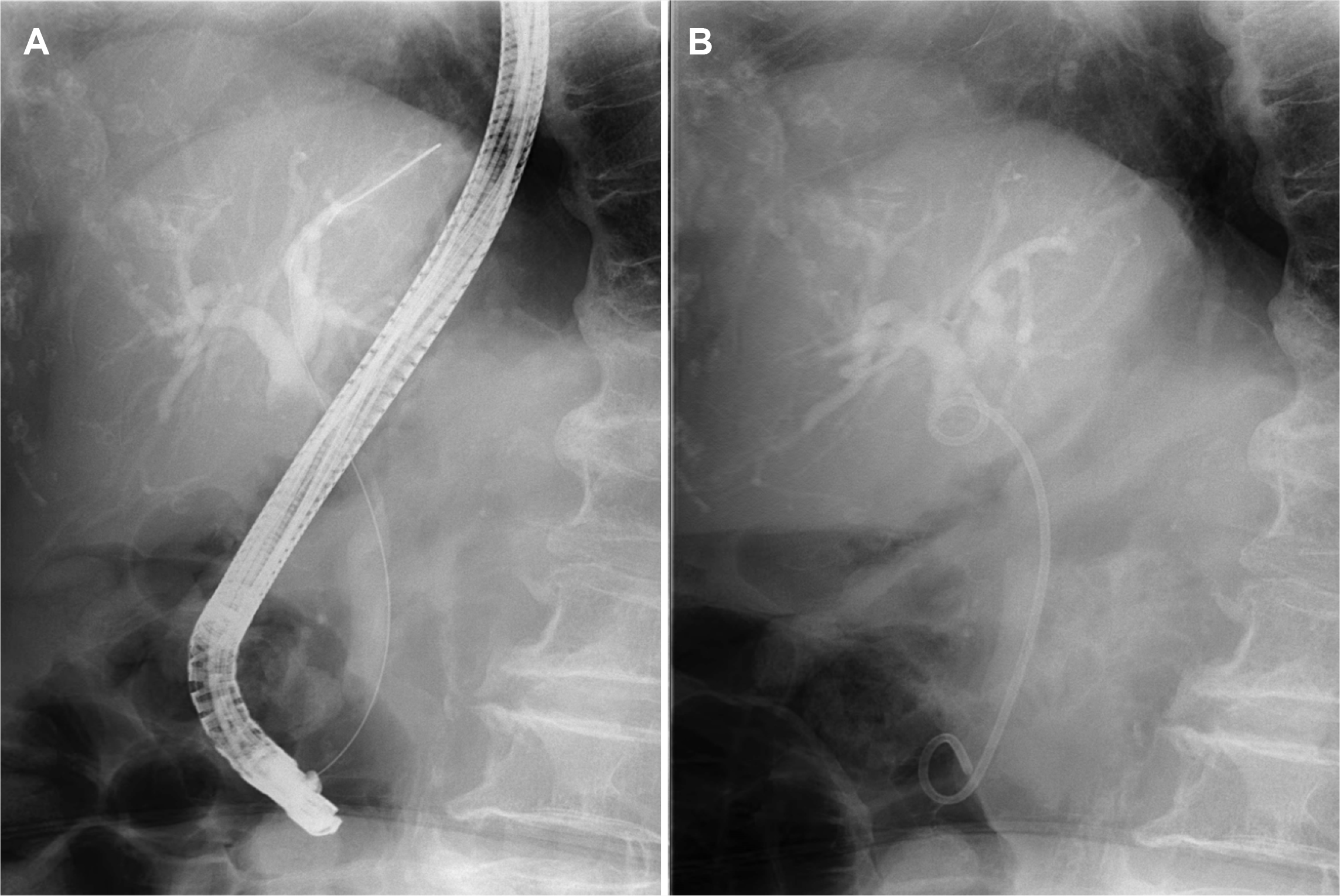
Fig. 3
(A) Histopathological examination shows solid and cord-structured proliferation of small tumor cells with of small tumor cells with irregularly shaped hyperchromatic nuclei (H&E, ×100). Immunohistochemical examinations show that the cells are positive for CD56 (B), synaptophysin (C), and a Ki-67 index of 85% (D) (IHC, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download