INTRODUCTION
Although pancreatic ductal adenocarcinoma (PDAC) is the fourteenth most common cancer worldwide, it is the seventh leading cause of cancer death.
1 In Korea, it is the
eighth most common cancer and the
fourth leading cause of cancer death.
2 A surgical resection is the only way to cure the disease, but the number of patients eligible for surgery at the initial diagnosis is less than 15%.
3 Thus, most patients are treated with systemic chemotherapy for palliative purposes.
3
Venous thromboembolism (VTE) is a frequent but under-recognized complication in patients with PDAC,
4 particularly in those receiving chemotherapy.
5,6 The incidence of VTE in patients with PDAC varies depending on the study population, follow-up duration, definition of VTE, and the methods used for diagnosing VTE.
4 The incidence ranged from 18% to 41.3% in retrospective Western cohorts.
5,7-10 On the other hand, several studies reported that it was low in Asians (5.3-18%).
11-15 In addition, it is unclear if the occurrence of VTE affects the prognosis. Many studies showed that VTE was associated with a poor overall survival (OS).
7,9,10,12,16. On the contrary, several studies reported that the occurrence of VTE was not associated with OS.
5,8,11,13,14
On the other hand, there are only a small number of studies on Korean patients, especially those with advanced PDAC who received palliative chemotherapy.
12,14,15 Therefore, this study examined the incidence of VTE in patients with advanced PDAC who received palliative chemotherapy. The risk factors associated with VTE and OS were also examined.
Go to :

DISCUSSION
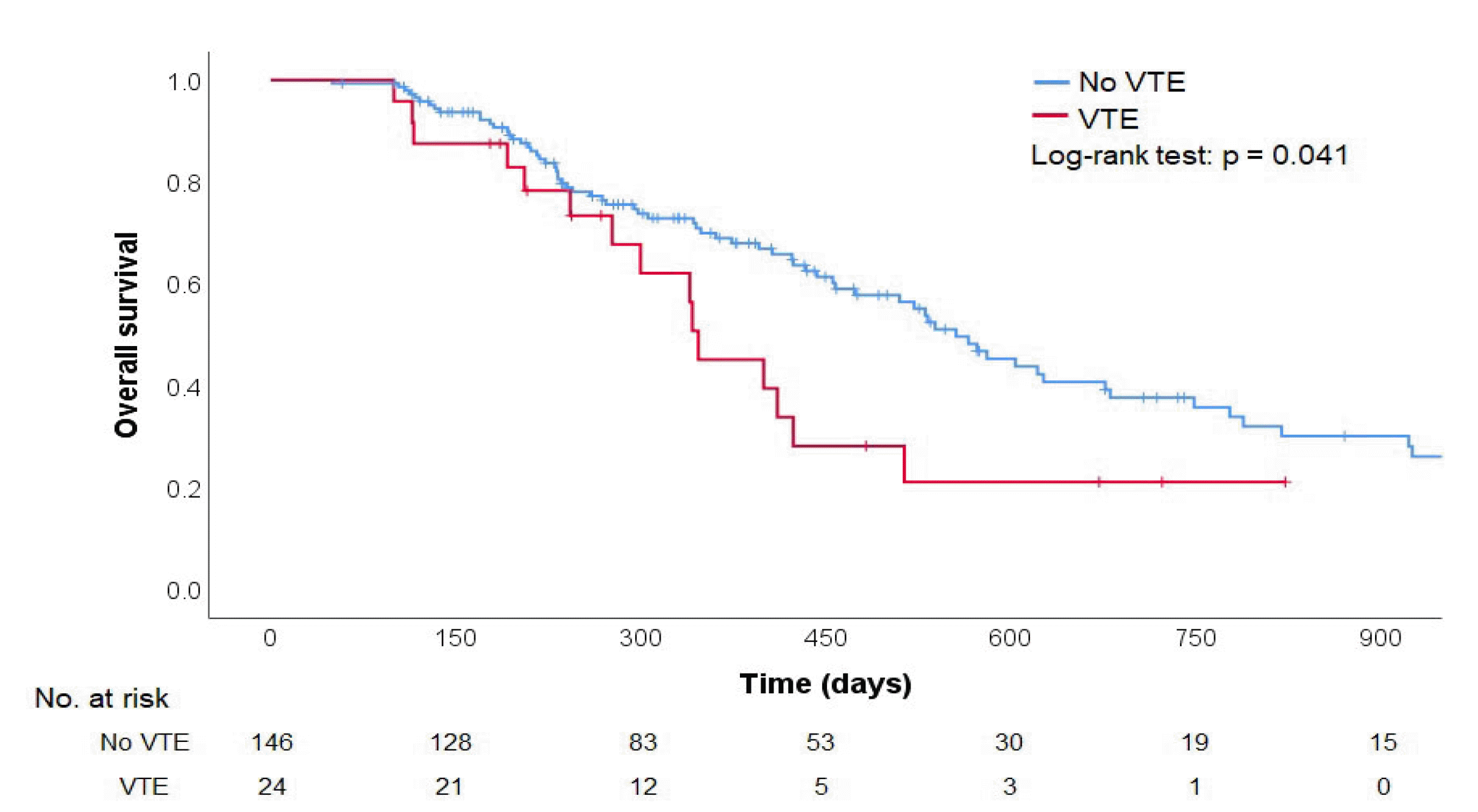
The cumulative incidence of VTE in patients with advanced PDAC who received palliative chemotherapy was 16.86% (95% CI, 11.50-24.36) at 360 days in this study. This result was lower than in previous studies in Western countries, where the incidence of VTE ranged from 18% to 41.3%.
5,7-10 On the other hand, a recent large prospective study reported that the cumulative incidence of VTE was 19.21% at 12 months, which was not significantly higher than the present result.
19 To the best of the authors’ knowledge, there have been no prospective studies on the incidence of VTE in patients with PDAC in Korea. Moreover, there have been only three retrospective studies. The reported incidences were 5.3%, 9.2%, and 18.6%, respectively.
12,14,15 VTE was not associated with a poor prognosis in these studies.
12,14 These are summarized in
Table 6. The difference in the results among the studies was probably attributed to the difference in the study population, such as the proportion of metastatic disease, surgical resection, and chemotherapy.
Table 6
Studies on the Incidence of Thromboembolism in Patients with Pancreatic Cancer in Korea
|
Parameters |
Lee et al.14
|
Yoon et al.12
|
Oh et al.15
|
Current study |
|
Study design |
Single center |
Single center |
Single center |
Single center |
|
Retrospective |
Retrospective |
Retrospective |
Retrospective |
|
Study period |
2005-2010 |
2006-2012 |
2003-2005 |
2011-2020 |
|
Incidence of VTE |
9.2%a
|
18.60% |
5.30% |
16.86%b
|
|
Disease stage |
|
|
|
|
|
Resectable |
316 (28.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Locally advanced |
191 (17.1) |
231 (45.7) |
25 (33.3) |
69 (40.6) |
|
Metastatic |
608 (54.5) |
252 (49.9) |
50 (66.7) |
101 (59.4) |
|
Recurrence |
0 (0.0) |
22 (4.4) |
0 (0.0) |
0 (0.0) |
|
Chemotherapy |
|
|
|
|
|
None |
216 (19.4) |
177 (34.3) |
35 (46.7) |
0 (0.0) |
|
Adjuvant |
173 (15.5) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Palliative |
689 (61.8) |
332 (65.7) |
40 (53.3) |
170 (100.0) |
|
VTE |
132 |
94 |
4 |
24 |
|
DVT |
17c
|
18 |
2 |
15 |
|
PTE |
38 |
19 |
0 |
16 |
|
SVT |
77 |
38 |
2 |
4 |
|
OS with or without VTE, months (p-value) |
9.5 vs. 9.3 (p=0.649) |
9.0 vs. 8.2 (p=0.237) |
NA |
11.6 vs. 18.5 (p=0.041) |

Cancer has been demonstrated to be an independent risk factor for VTE. Moreover, PDAC carries the highest risk for VTE.
4,18 One possible explanation is that tissue factor-positive microvesicles released from cancer provide a surface for assembling different coagulation factor complexes.
20 The endocrine function of the pancreas provides an easy route for transporting tissue factor-positive microvesicles from a tumor to the blood.
20 In this study, a CA 19-9 level over 1,000 U/mL was a significant risk factor for VTE. The CA 19-9 level is a well-known prognostic factor for PDAC and reflects the disease burden.
21 CA 19-9 was associated with thrombin generation in treatment-naïve patients with PDAC.
22 The location of the primary tumor is also a well-known risk factor for VTE, which has been commonly identified in several previous studies.
7,8,14,19 Most reported that body or tail cancer had a higher risk of VTE than head cancer.
7,8,19 On the other hand, in this study, the tumor location was not a statistically significant factor, even though pancreatic body cancer had a marginally protective effect according to multivariate analysis. Frere et al.
19 reported that the tumor location was not a statistically significant factor when VVT was excluded from the analysis. Hence, it is probably because the proportion of VVT in this study was only 16.7%, which contrasts with other studies: 29.6-58.3%.
7,8,14,19
Interestingly, a history of alcohol consumption was a protective factor for VTE in this study. Several studies reported that moderate alcohol consumption is associated with a decreased risk of VTE.
23-25 Light-to-moderate alcohol consumption was associated with lower levels of coagulation factors, including fibrinogen, von Willebrand factor, and factor VII.
26 On the other hand, a detailed history of alcohol consumption could not be obtained because of its retrospective design, and alcohol consumption probably decreased after the diagnosis of PDAC. Thus, further prospective studies are needed to investigate the potential protective role of alcohol against VTE in patients with advanced PDAC.
In this study, although the disease stage of patients did not change significantly at VTE diagnosis, 45.8% of patients had been diagnosed with disease progression. This rate was comparable to the result of previous studies, which reported that the proportion of patients with progressive disease at the time of VTE diagnosis ranged from 29.4% to 57.1%.
12,16,27 In addition, the hemoglobin level was lower, and the PT was prolonged during VTE diagnosis. Nevertheless, anemia is a common hematologic adverse event in patients with PDAC who are undergoing chemotherapy.
28 Moreover, prolonged PT reflects the biosynthetic capacity of the liver; hepatic insufficiency caused by disease progression may lead to a decreased coagulation factor.
29 PT prolongation was associated with disease progression in other cancers, particularly liver metastasis.
29,30 Therefore, it is difficult to attribute these changes solely to the development of VTE.
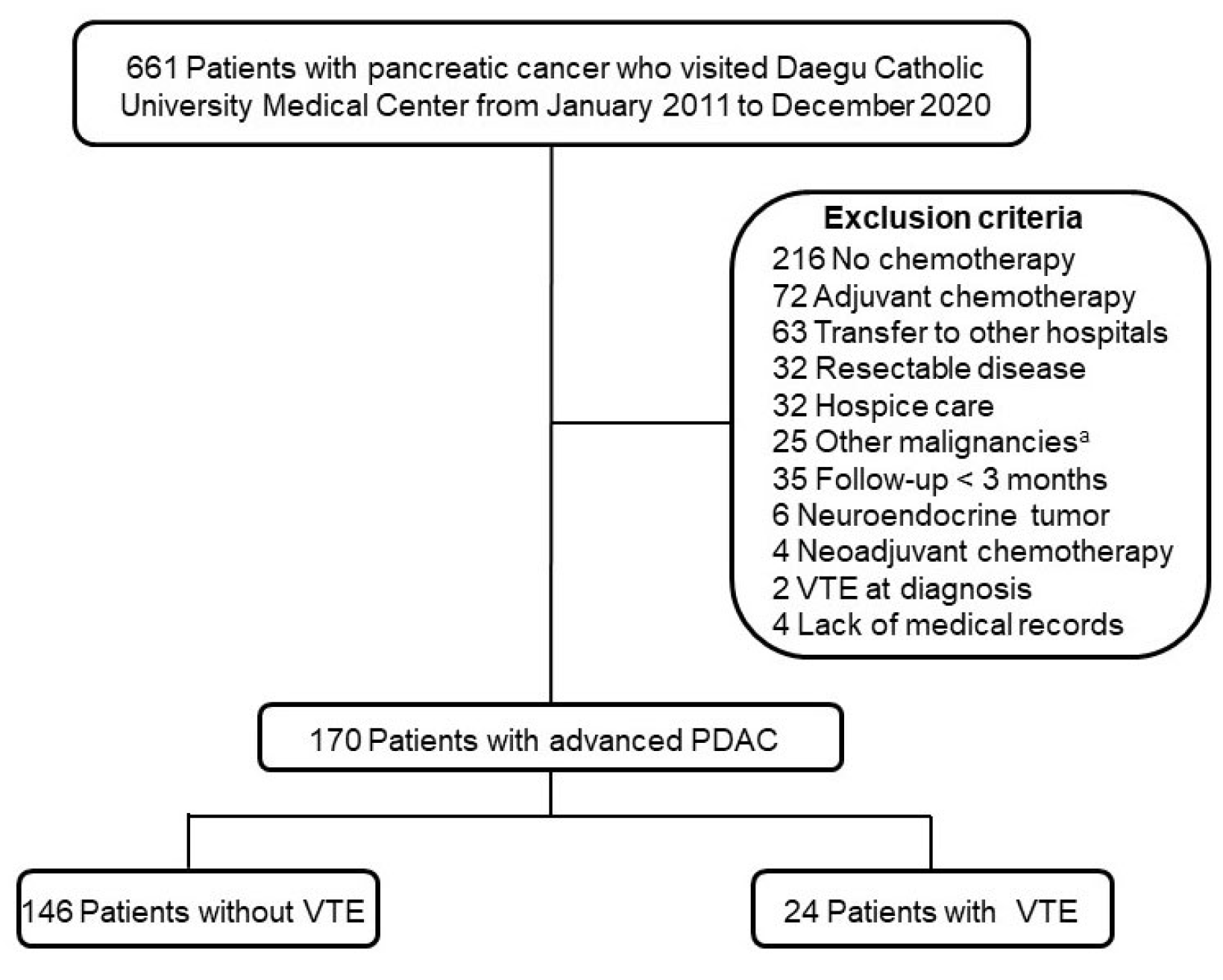
Patients with VTE showed significantly reduced OS in this study. In addition, CA 19-9 level and VTE were independent prognostic factors in multivariate analysis. The elevation of the CA 19-9 level is a well-known prognostic factor in previous studies.
14,16 In contrast, VTE has shown inconsistent results in previous studies. Several studies have shown that the occurrence of VTE is a predictor of a poor prognosis,
10,12,19 particularly in early VTE, which was defined as within 1.5 months after the initiation of chemotherapy or within 3 months after diagnosis of PDAC.
7,9,13,16 Other studies reported that the occurrence of VTE does not significantly affect the prognosis.
5,8,11,14 As mentioned above, this is probably explained by the difference in the study population among studies. Berger et al.
5 reported that VTE was not associated with a poor prognosis in patients with advanced PDAC who received palliative chemotherapy. On the other hand, the study population had a higher proportion of metastatic disease than the present study (92.0% vs. 59.4%) and had shorter median survival in the no VTE group (9.9 months vs. 347 days).
5
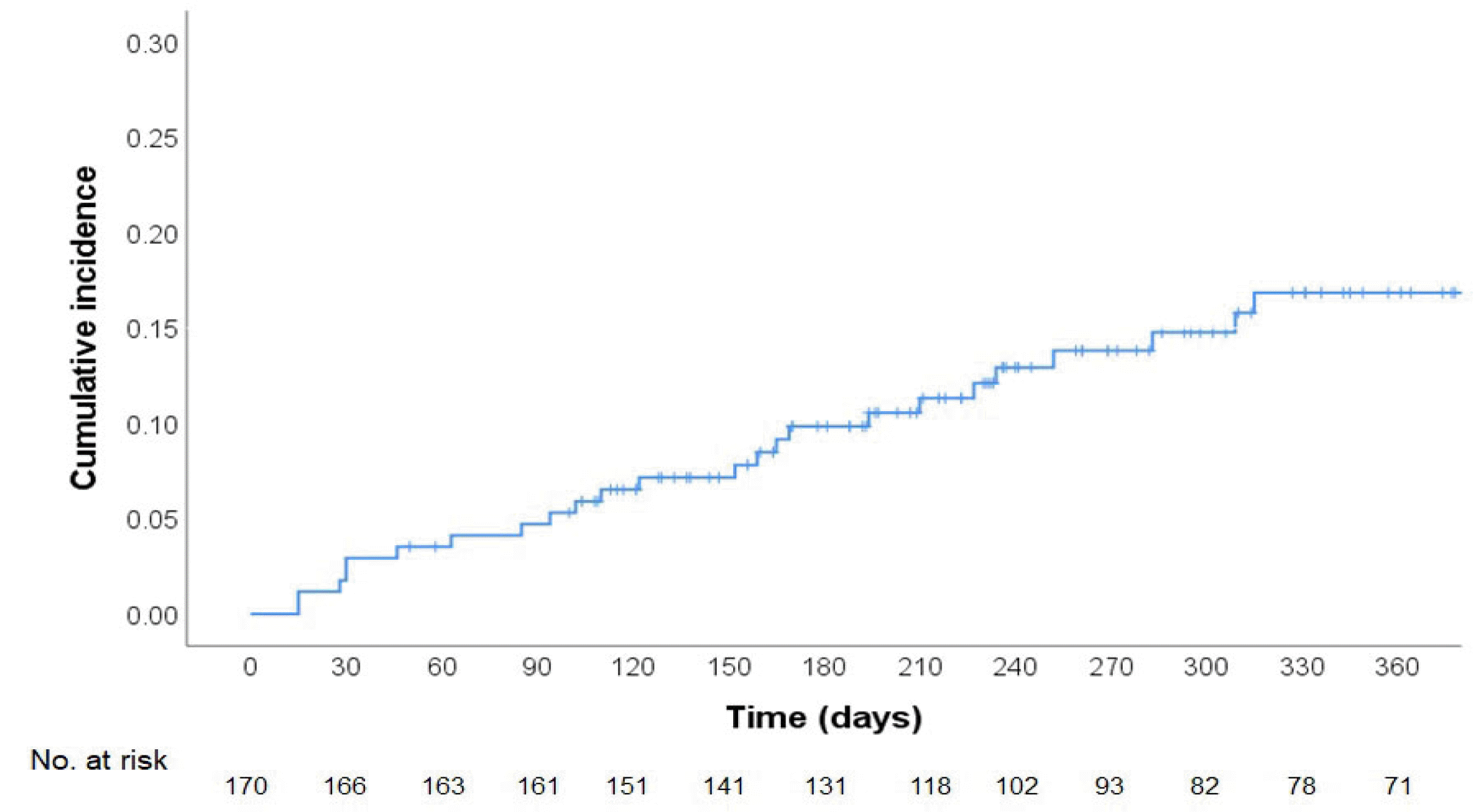
Regarding symptoms, the proportion of incidental VTE was 50% in this study, similar to the 52.4% to 72% reported elsewhere.
7,8,10,12,14,19 In contrast, there have been conflicting results as to whether symptomatic VTE has a poorer progress than incidental VTE.
7,10,14,19 The data showed no significant difference with respect to OS according to the presence or absence of symptoms.
To the best of the authors’ knowledge, there have been five large prospective studies to evaluate the efficacy of prophylactic anticoagulation. Among them, two studies were conducted on only patients with advanced PDAC.
31,32 Although thromboprophylaxis decreased the risk of VTE in these studies significantly, there was no survival gain. Therefore, extensive prospective studies on thromboprophylaxis in patients with the risk factors for VTE are needed.
This study had some limitations. First, it was a retrospective single-center study. Thus, there was selection bias. In addition, it is difficult to generalize the results of this study. Second, the history of alcohol consumption, an important protective factor for VTE in this study, was not obtained in detail because of a lack of medical records. Third, 48.2% of patients were diagnosed by EUS-FNA because EUS-FNB had not been performed before 2017 at the authors’ institution. Therefore, it was not possible to obtain enough samples to evaluate the tumor differentiation. Fourth, the risk factors affecting the occurrence of VTE were not evaluated thoroughly because of the retrospective design and lack of medical records. These factors included recent surgery, neurological diseases, performance status, and medications, such as oral contraceptives, anticoagulants, and antiplatelet agents. On the other hand, because the patients in this study included those who had received chemotherapy, they were neither bedridden nor in a poor performance status. Finally, a chest CT scan was performed only when needed. Thus, the incidence of VTE might be underestimated. Nevertheless, regular follow-up was performed during chemotherapy because the study was conducted on only patients who received palliative chemotherapy. Therefore, the incidence of VTE in patients in this study with PDAC is probably close to the actual value.
In conclusion, the cumulative incidence of VTE in patients with advanced PDAC who received palliative chemotherapy was 16.9% (95% CI, 11.50-24.36) at 360 days. Although a history of alcohol consumption was a protective factor, a high CA 19-9 level was a risk factor for VTE. Furthermore, the occurrence of VTE was associated with a poor prognosis. On the other hand, because there has been no prospective study on Asian patients, a further large prospective study will be needed to investigate the incidence and risk factors associated with VTE.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download