Abstract
A Clostridioides difficile infection (CDI) is one of the major nosocomial diarrheal diseases. Pseudomembranous colitis (PMC) is a characteristic endoscopic finding of CDI, manifested by white or yellowish plaque covering the colonic mucosa. Ischemic colitis is inflammation of the colon manifested by mucosal denudation and friability. Ischemic colitis is rarely associated with CDI. The treatment response might be delayed when CDI is complicated with other diseases that cause diarrhea. Thus far, reports of CDI concomitant with Cytomegalovirus (CMV) colitis are rare. This paper reports a case of PMC and ischemic colitis associated with CDI and CMV infection. After two weeks of oral vancomycin and intravenous metronidazole, the patient’s diarrhea was not improved. Follow-up sigmoidoscopy was performed, and a CMV infection was identified at areas of broad ulceration where ischemic colitis occurred. Finally, the patient was cured with ganciclovir. Follow-up sigmoidoscopy showed an improvement in ischemic colitis.
Clostridioides difficile (C. difficile) infection (CDI) is a common nosocomial diarrhea induced by disruption of intestinal microbiota. Pseudomembranous colitis (PMC) is a characteristic endoscopic finding of CDI, manifested by white or yellowish plaques on the colonic mucosa.1 Ischemic colitis is inflammation of the colon manifested by mucosal denudation and friability.2 Ischemic colitis is rarely associated with CDI.3 Thus far, CDI concomitant with cytomegalovirus (CMV) colitis has rarely been reported. This paper presents a case of PMC concomitant with ischemic colitis caused by a CDI and CMV infection that was treated successfully with oral vancomycin, intravenous metronidazole, and ganciclovir.
An 82-year-old female visited the authors’ hospital complaining of aphagia. Her vital signs and laboratory findings at the initial presentation were as follows: blood pressure, 110/65 mmHg; pulse rate, 85 beats/min; body temperature, 36.5°C; hemoglobin, 11.9 g/dL, white blood cell count, 8,200 cells/mm3; C-reactive protein, 5.8 mg/dL; albumin, 2.9 g/dL; and creatinine, 1.26 mg/dL. The patient was diagnosed with a middle cerebral artery infarction and treated with aspirin and clopidogrel. During hospitalization, the patient developed pneumonia and was treated with broad-spectrum antibiotics. Diarrhea (five times/day) and hematochezia developed 10 days after antibiotic treatment.
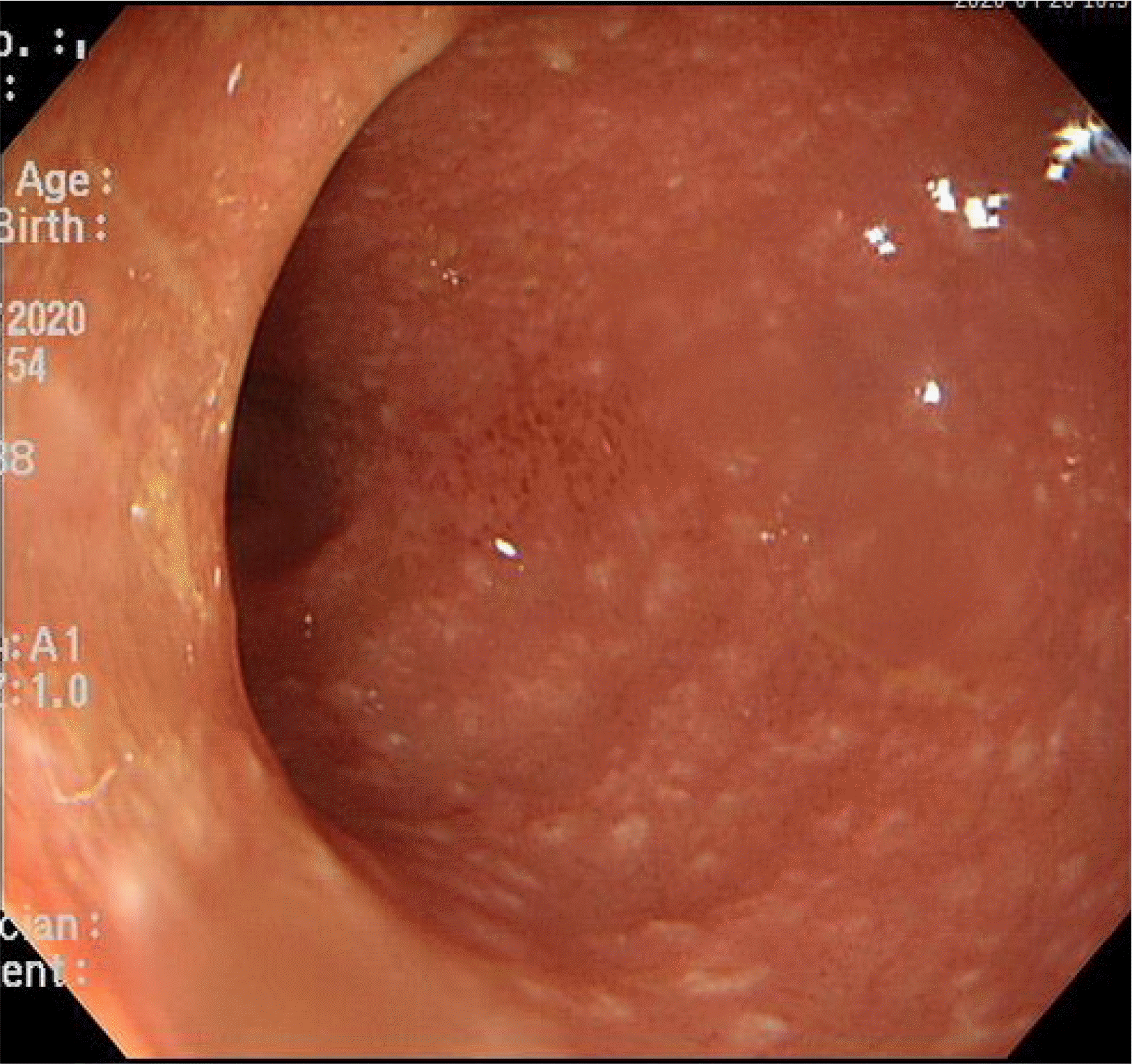
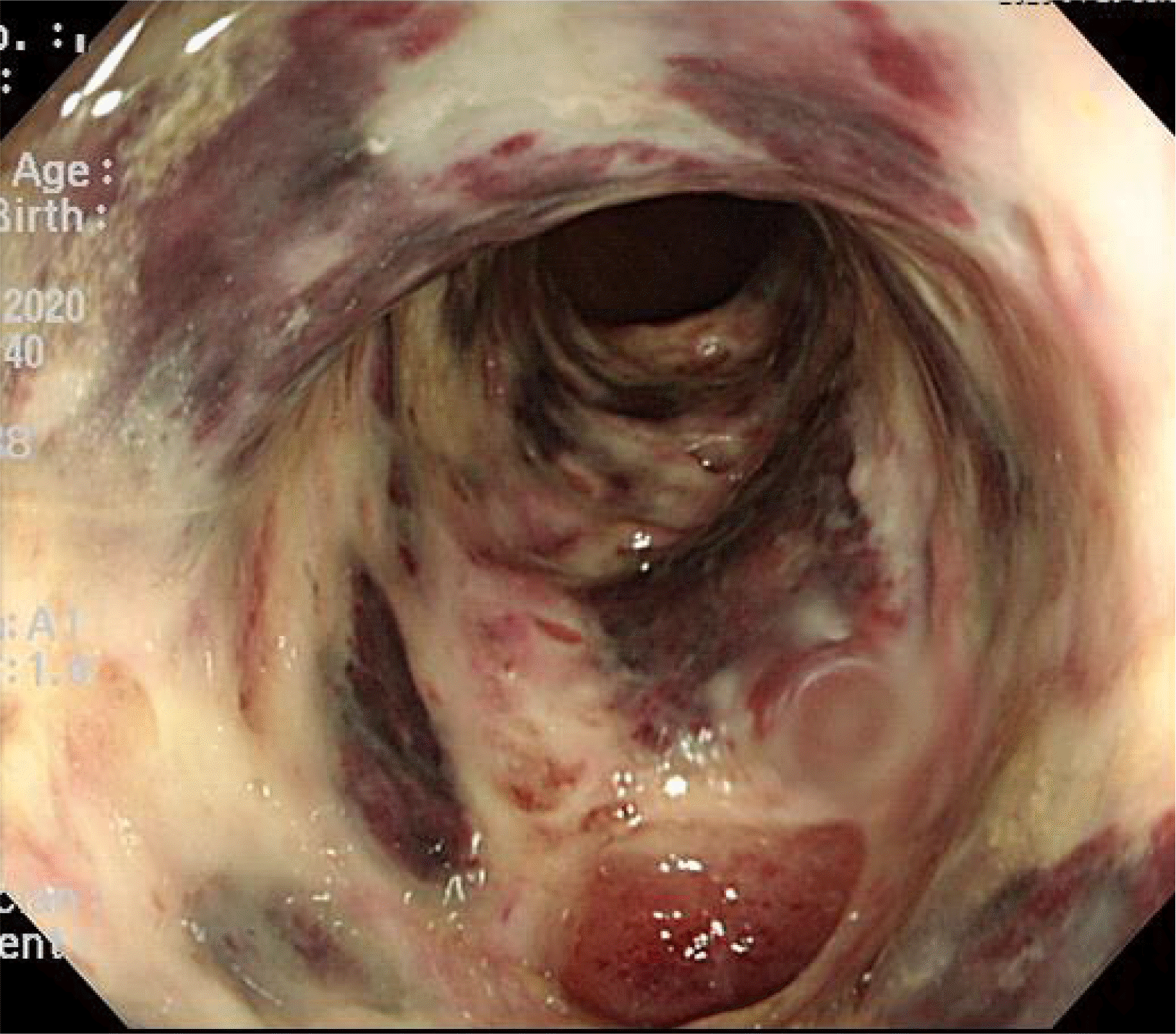
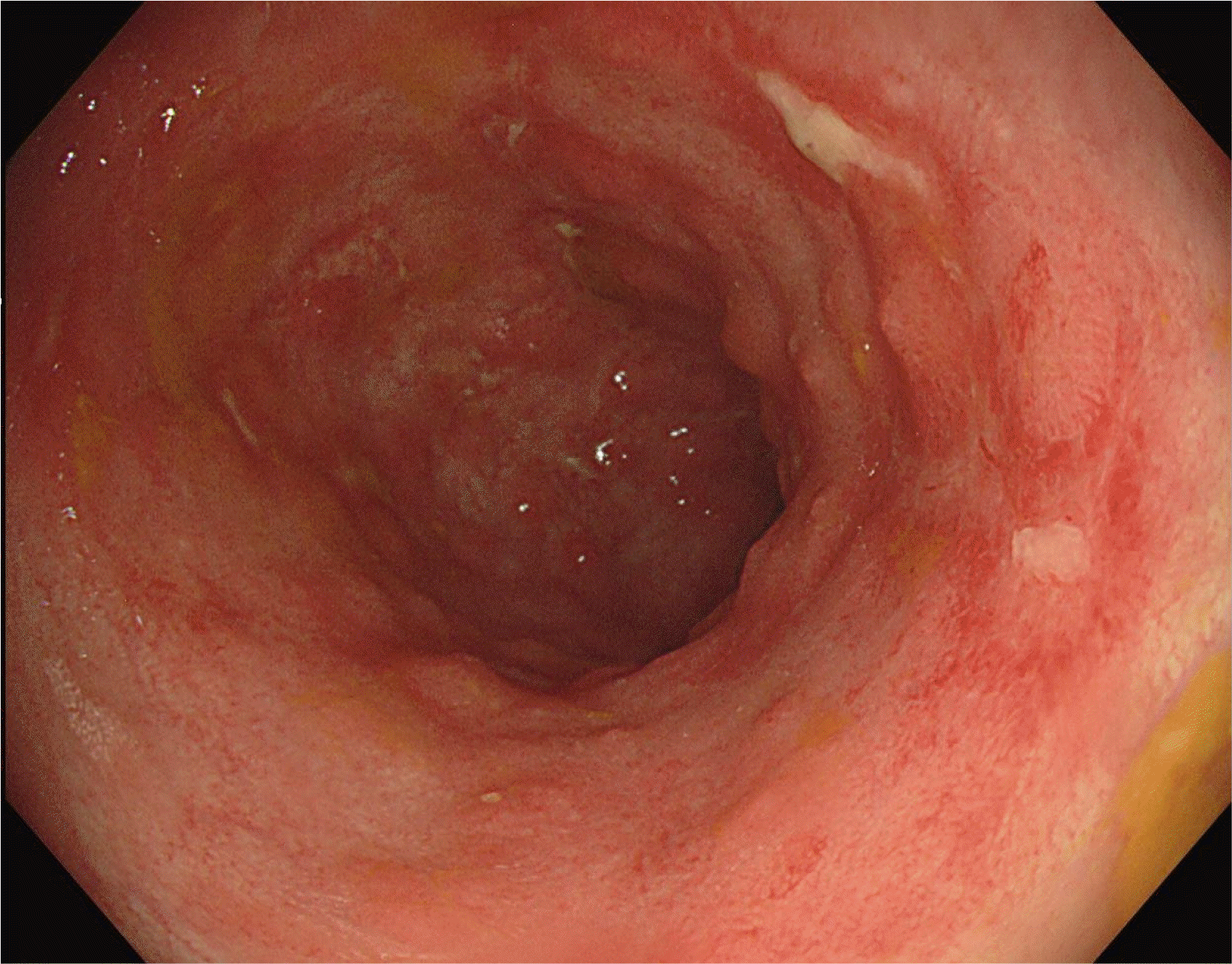
Sigmoidoscopy showed multiple tiny yellowish plaque in the sigmoid colon (anal verge, 35–45 cm), compatible with PMC (Fig. 1). Together with PMC, extensive mucosal denudation and colonic necrosis, compatible with ischemic colitis, were found at the anal verge 20–35 cm, close to the areas of PMC (Fig. 2). The mucosa was very friable. A stool study was performed. C. difficile toxin was detected. The PCR for toxigenic C. difficile was positive in stool samples. Oral vancomycin (500 mg, four times/day) and intravenous metronidazole (500 mg, three times/day) were started because the patient’s symptoms were believed to have been compatible with fulminant CDI. Broad-spectrum antibiotics for pneumonia were stopped because her pneumonia had resolved.
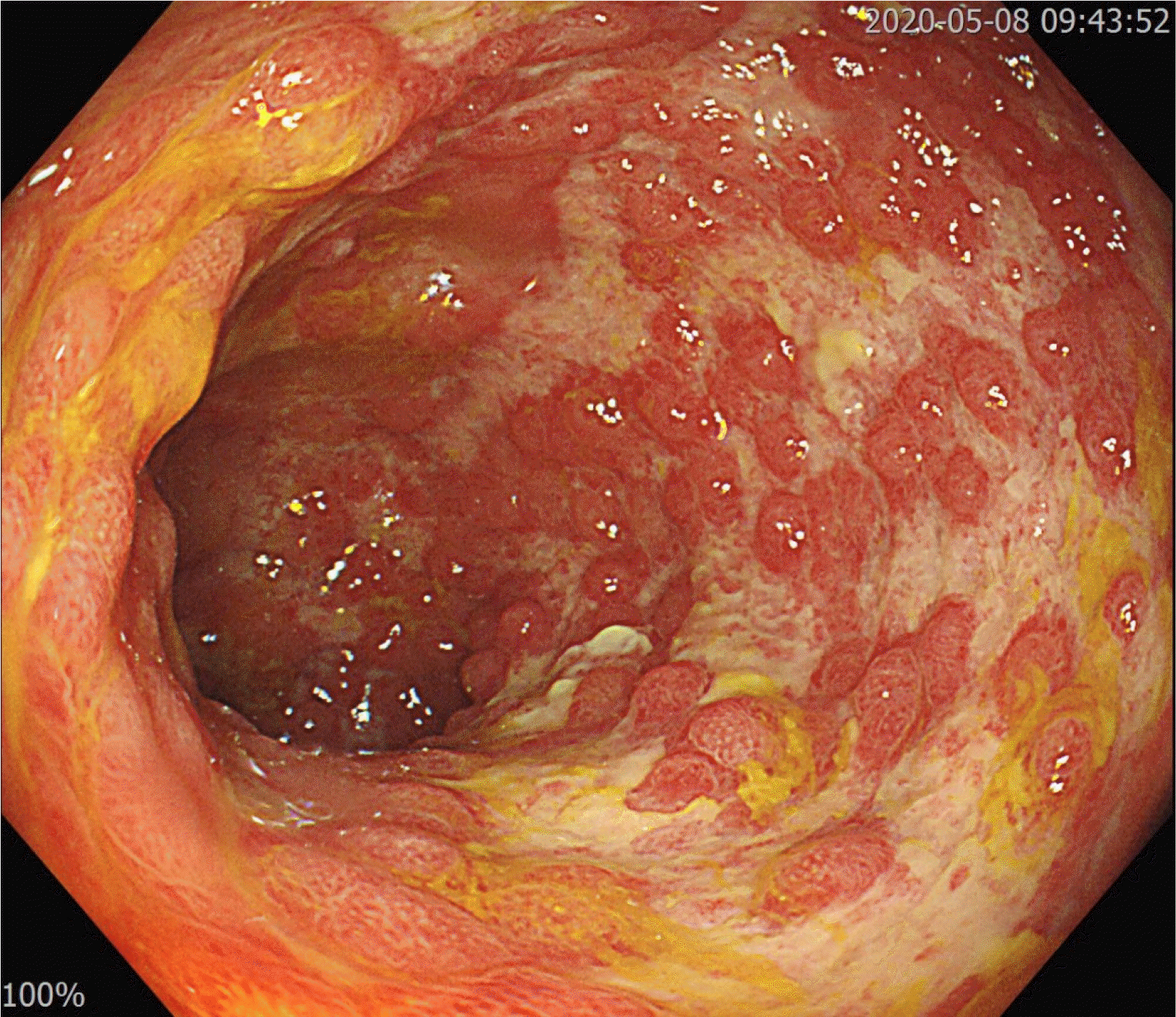
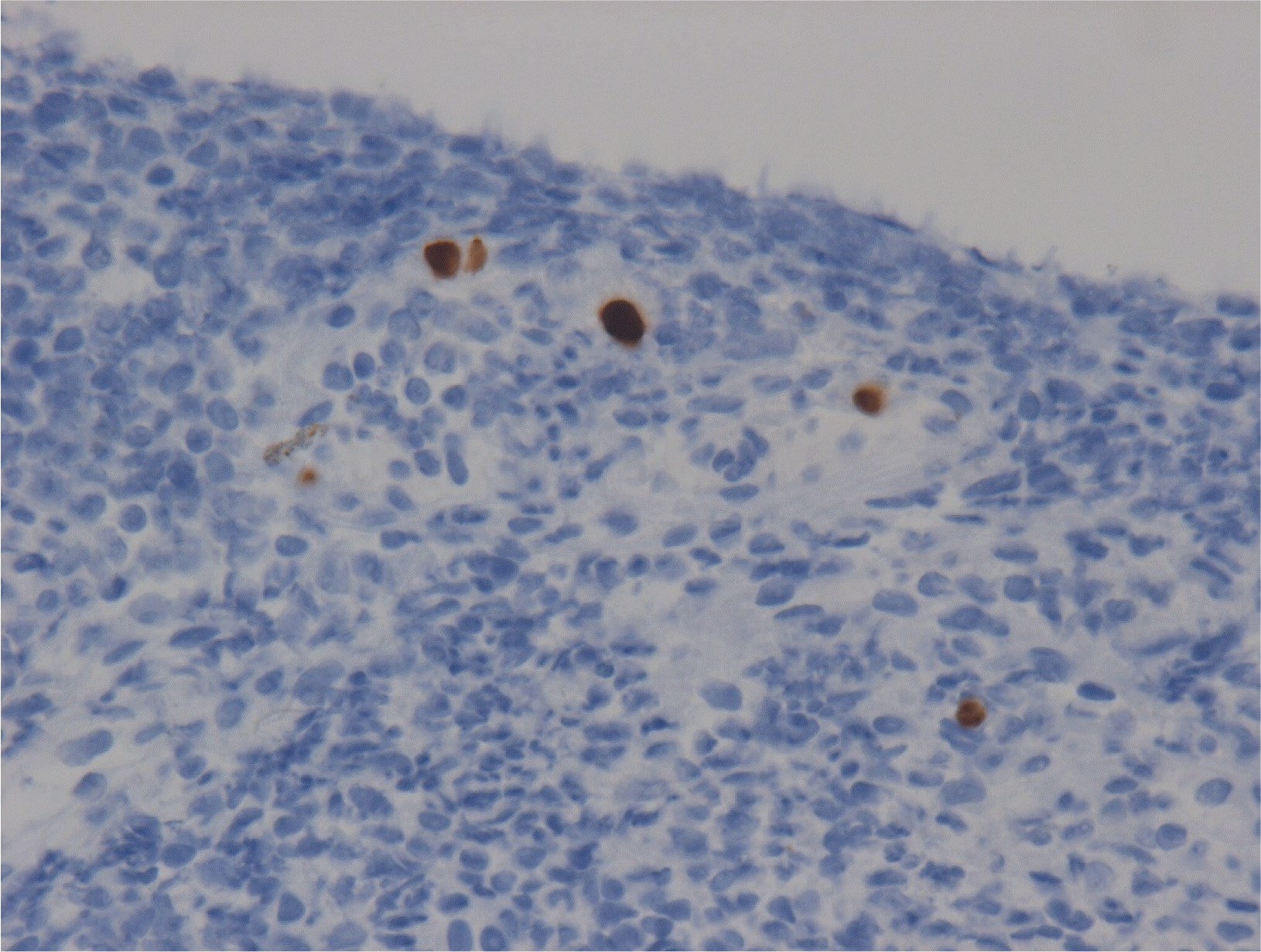
Ten days after CDI treatment, hematochezia stopped, but the frequency and volume of diarrhea were not decreased. Her body temperature varied between 37.0°C and 38.5°C over 10 days. A stool study for C. difficile and follow-up sigmoidoscopy were performed. Sigmoidoscopy, performed 10 days after CDI treatment, revealed the resolution of PMC. On the other hand, broad ulceration was still noted where ischemic colitis had occurred (Fig. 3). Multiple tiny mucosal edema mimicking pseudopolyps were found on the surface of the ulceration. A forceps biopsy with a CMV stain was performed. The biopsy results were consistent with a CMV infection (Fig. 4). On the follow-up stool study, C. difficile toxin was not detected, but C. difficile by PCR was still positive. Ganciclovir, 300 mg twice daily, was started, and CDI treatment was continued. After seven days of ganciclovir treatment, the frequency of diarrhea decreased to one–two times per day, and her fever subsided. Anti-CDI treatment was stopped (total treatment duration, 18 days). The diarrhea resolved after 14 days of ganciclovir treatment. Follow-up sigmoidoscopy showed improvement of widespread colonic ulceration (Fig. 5), while small foci of ulceration with hyperemia were found. Ganciclovir was used for three weeks, and the patient was discharged on hospital day 42.
Informed consent was waived because this case report was done in retrospective manner.
This paper describes a case of CDI concomitant with CMV colitis, manifested by PMC and ischemic colitis. CDI likely caused the PMC, and the CMV infection caused the ischemic colitis. The patient was cured using an anti-CDI treatment and ganciclovir.
Multiple yellowish plaques are a typical endoscopic finding in PMC. Consistent with ischemic colitis, mucosal denudation, friability, and necrosis were observed close to the PMC. Although ischemic colitis associated with CDI has rarely been reported,4 it was initially believed that the ischemic colitis was associated with CDI because the stool study was compatible with CDI. Ischemic colitis was found close to the PMC. Because the initial sigmoidoscopic finding showed severe colitis, This study did not consider a forceps biopsy of the inflamed colon, expecting that a biopsy of the severely inflamed area might yield only necrotic tissue.
Most guidelines recommend that anti-CDI treatment be performed for at least 10 days.1,5 There is no consensus on the timing of the reevaluation of patients with CDI. The patient’s diarrhea did not improve despite a 10-day course of anti-CDI treatment. Although repetition of the stool study was not recommended, the patient was reevaluated using both the stool study and sigmoidoscopy because the patient was not improving. Colitis was still severe at follow-up, and a CMV infection was discovered. Although CMV colitis is prevalent in immunocompromised patients, it can be complicated even in immunocompetent patients.6,7
In immunocompromised patients, such as those with inflammatory bowel disease (IBD), few articles have been published regarding the coinfection of CDI and CMV. For IBD patients, the use of antibiotics and hospitalization are classic risk factors for CDI, while CMV infection is an independent risk factor for CDI.8
There is no accepted definition of refractory CDI unresponsive to standard treatment. Only one guideline defines refractory CDI as CDI not responding to standard treatment after three–five days of therapy.9 Before the diagnosis of CMV infection, the patient’s status was considered compatible with refractory CDI. Fecal microbiota transplantation (FMT) is indicated for the treatment of refractory CDI. On the other hand, FMT should be indicated only after other causes of diarrhea have been excluded. In the present case, the CMV infection was a hidden cause of persistent diarrhea after receiving CDI treatment. The patient gradually improved after treatment of CDI and CMV colitis.
This paper described a rare case of CDI concomitant with CMV colitis manifested by PMC and ischemic colitis in an immunocompetent patient. A CMV infection, which was initially not diagnosed, was the cause of ischemic colitis and persistent diarrhea. Other causes of diarrhea should be considered in refractory CDI, especially in cases of severe colitis. This report suggests reevaluating patients with refractory CDI unresponsive to standard treatment.
Notes
REFERENCES
1. Kelly CR, Fischer M, Allegretti JR, et al. 2021; ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am J Gastroenterol. 116:1124–1147. Erratum in: Am J Gastroenterol 2022;117:358. DOI: 10.14309/ajg.0000000000001278. PMID: 34003176.
2. Trotter JM, Hunt L, Peter MB. 2016; Ischaemic colitis. BMJ. 355:i6600. DOI: 10.1136/bmj.i6600. PMID: 28007701.
3. Moulis H, Vender RJ. 1994; Antibiotic-associated hemorrhagic colitis. J Clin Gastroenterol. 18:227–231. DOI: 10.1097/00004836-199404000-00012. PMID: 8034921.
4. Kang SH, Gweon TG, Hwang H, Baeg MK. A case of ischemic colitis complicated by clostridioides difficile infection treated with fecal microbiota transplantation. Clin Endosc. 2022; Jan. 20. doi: 10.5946/ce.2021.199. DOI: 10.5946/ce.2021.199.
5. McDonald LC, Gerding DN, Johnson S, et al. 2018; Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 66:987–994. DOI: 10.1093/cid/ciy149. PMID: 29562266.
6. Le PH, Lin WR, Kuo CJ, et al. 2017; Clinical characteristics of cytomegalovirus colitis: a 15-year experience from a tertiary reference center. Ther Clin Risk Manag. 13:1585–1593. DOI: 10.2147/TCRM.S151180. PMID: 29290686. PMCID: PMC5735984.
7. Yeh PJ, Wu RC, Chiu CT, et al. 2022; Cytomegalovirus diseases of the gastrointestinal tract. Viruses. 14:352. DOI: 10.3390/v14020352. PMID: 35215942. PMCID: PMC8879032.
8. Xu H, Tang H, Xu T, et al. 2019; Retrospective analysis of Clostridium difficile infection in patients with ulcerative colitis in a tertiary hospital in China. BMC Gastroenterol. 19:3. DOI: 10.1186/s12876-018-0920-x. PMID: 30616563. PMCID: PMC6323708.
9. van Prehn J, Reigadas E, Vogelzang EH, et al. Guideline Committee of the European Study Group on Clostridioides difficile. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021; 27 Suppl 2:S1–S21. DOI: 10.1016/j.cmi.2021.09.038. PMID: 34678515.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download