Abstract
Background and Objectives
This study aimed to investigate the protective effect of polydeoxyribonucleotide (PDRN) against skin flap necrosis in a murine skin flap model.Materials and Method Twenty mice with rectangular skin flaps on the dorsum were randomly divided into the PDRN (n=10) and pentobarbital sodium (PBS) (n=10) injection groups. PDRN (8 mg/kg) was subdermally injected at 12 different points immediately after the operation. After 7 days, the flap perfusions were evaluated using a laser speckle contrast imaging (LSCI) system, and specimens were collected for immunohistochemistry analysis.
Results
The percentage of survival area relative to the total flap area was significantly higher in the PDRN group (60.87%±7.63%) than in the PBS group (45.23%±10.72%) (p<0.05). The mean LSCI perfusion signal of the distal part of the skin flap in the PBS group was 0.57±0.12, and that in the PDRN group was 0.74±0.13 (p<0.05). The PDRN group had a significantly lower interleukin 1 beta expression than the PBS group and higher vascular endothelial growth factor α expression than the PBS group (p<0.05).
Random skin flaps are perfused from a musculocutaneous or septocutaneous vessel and connect to the subdermal plexus through a thin, unnamed peripheral artery that supplies blood [1]. Random skin flaps can be classified as local flaps based on how the flap moves. They can be safely used on the face where there is an abundance of subdermal plexus [2]. As the random skin flaps used to reconstruct defects are made of the same tissue as the defect site, they have similar color and texture as the original skin after surgery. These skin flaps reduce donor site complications, as the reconstruction can be performed under a single surgical field of view. However, reconstruction with a random skin flap is difficult for large defects, and necrosis of the distal part of the flap may occur if the flap lacks a sufficient blood supply [3,4]. Ischemic necrosis of the distal flap is the most common and lethal complication of flap surgery and occurs when angiogenesis does not occur laterally from the base of the flap [5]. The clinical effectiveness of various medication treatments used to prevent ischemic necrosis remains controversial [6-8].
Polydeoxyribonucleotide (PDRN) is a DNA fragment extracted and processed from salmon sperm DNA that has 95% similarity to human DNA and tissue regeneration and anti-inflammatory properties [9]. This study investigated the protective effect of PDRN against ischemic necrosis through the injection of PDRN into random skin flaps on the backs of mice.
This study was approved by the Institutional Animal Care and Use Committee of the Yonsei University Wonju College of Medicine (Protocol YWC-190425-1). Seven-week-old male Balb-c/nu mice (Jackson Laboratories, Bar Harbor, ME, USA) were acclimatized for 7 days and maintained in an air-conditioned specific-pathogen-free room at 21°C with a light/dark cycle of 12 hours/12 hours. To induce anesthesia, pentobarbital sodium (PBS) (20 mg/kg body weight) was intraperitoneally injected into nude mice. A 2×4 cm rectangular flap was designed on the dorsal skin of the mice (Fig. 1A). A caudally based flap elevation, including the elevation of the panniculus carnosus muscle, was performed (Fig. 1B). A silicone sheet was inserted between the flap and dorsal muscle to stop the blood supply, and the skin was sutured with nylon 5-0 sutures. PDRN (8 mg/kg) was subdermally injected at 12 different points across the proximal, middle, and distal parts of the flap in mice in the experimental group (n=10). In the control group (n=10), PBS was injected at the same locations and at the same concentration as PDRN. The solutions were injected immediately postoperatively.
The surgical sites were examined each day grossly to determine the degree of skin necrosis. On postoperative day 7, the mice were anesthetized using inhalation anesthesia, and images of the flaps were obtained. The Image J software program (National Institute of Health, Rockville, MD, USA) was used to determine the percentage of the survived tissue area relative to the total flap area. A laser speckle contrast imaging (LSCI) device developed by Jung’s group was used to quantitatively analyze flap perfusion [10]. The flaps were divided into proximal, middle, and distal parts, and the ratio of the perfusion signal of the distal part to the perfusion signal of the normal skin was compared. A perfusion signal of 1 was assigned to normal skin.
The mice were euthanized on postoperative day 7, and tissue was collected from the surgical site. The collected tissue was fixed in 10% formalin for 24 hours, washed in X-wash for 12 hours, and dehydrated in an automatic tissue processor for clearing. The tissue was embedded in paraffin and sectioned into 4-μm-thick blocks. Tissue slices were deparaffinized with xylene, rehydrated with ethanol, treated with hydrogen peroxide methanol for 10 minutes to block endogenous peroxidase, and washed with distilled water followed by 50 mM Tris Buffer. Subsequently, the samples were treated with goat serum for 30 minutes. After the remanent solution was removed from the slides, they were treated with vascular endothelial growth factor α (VEGFα; Affinity, Changzhou, China) and interleukin 1 beta (IL-1β; Affinity, Changzhou, China) primary antibodies at 4°C for 12 hours. Thereafter, the slides were washed three times with Tween/Tris-buffered saline (TTBS), treated with secondary antibodies (which were the same as the primary antibodies) for 30 minutes, and washed three times with TTBS for 5 minutes. The slides were developed using ImmPACT DAB substrate kit (Vector, Burlingame, CA, USA) for 1 minute and washed with distilled water for 5 minutes. Following the wash, the slides were stained with hematoxylin and washed with distilled water. Then, the slides were treated with 1% acid alcohol once or twice, washed for 5 minutes, treated with 1% ammonia water, and washed for 3 minutes. The slides were dehydrated with 70%-100% alcohol for 1 minute at each alcohol concentration, treated with xylene for 3-5 minutes, and mounted on a cover glass. Once staining was complete, the slides were analyzed using a Slide Scanner (Motic, San Francisco, CA, USA). Solution for Automatic Bio-Image Analysis software (ebiogen, Seoul, Korea) was used to quantitatively analyze the stained tissues. IL-1β expression was analyzed to examine the differences in inflammatory responses in flaps between the two groups. The level of IL-1β expression was compared by measuring the area of the IL-1β-positive epidermis in the stained tissue. VEGFα expression was analyzed to investigate the mechanisms of necrosis protection provided by PDRN. The level of VEGFα expression was determined by counting the cells deemed VEGFα-positive per unit area of the epidermis in the stained tissue.
A two-way analysis of variance test was used to compare the wounded areas between the groups. The Kruskal-Wallis test was used to analyze the results of the histological examinations. Statistical significance was set at p<0.05. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, NY, USA).
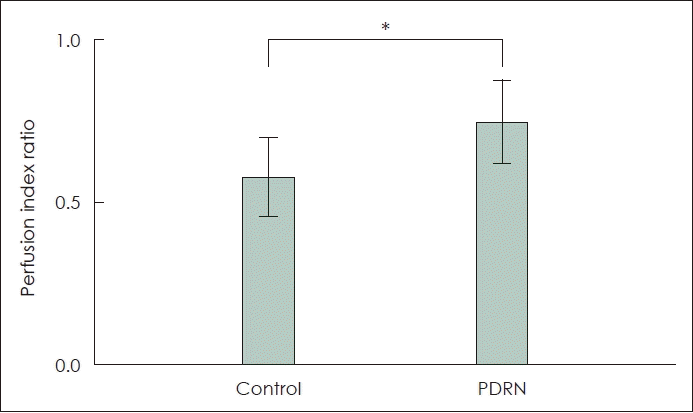
All 20 mice survived until postoperative day 7 with no flap loss due to infection or cannibalism. Postoperative flap necrosis began at the distal end of the graft. On postoperative day 7, the percentage of surviving flap tissue area relative to the total flap area was significantly higher in the experimental group (60.87%±7.63%) than in the control group (45.23%±10.72%) (p<0.05) (Fig. 2A and D). In the control group, necrosis was observed along the inner surface of the flaps, as was swelling and blood congestion (Fig. 2F). No significant skin discoloration was observed in the experimental group, and the vessels maintained their shape (Fig. 2C). The mean LSCI perfusion signal of the distal part of the skin flap in the control group was 0.57±0.12, and that in the experimental group was 0.74±0.13 (p<0.05) (Figs. 2B, 2E, and 3).
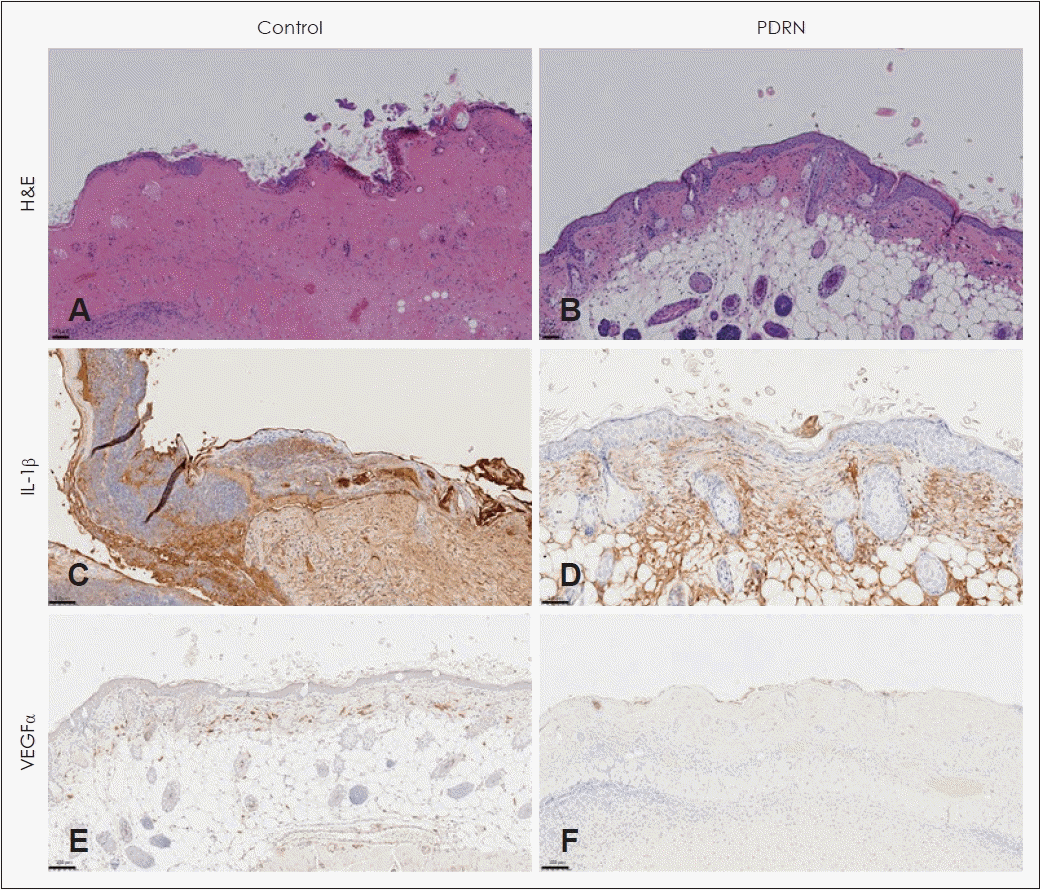
Histologic analysis revealed skin lacerations resulting from skin necrosis in the control group. Necrosis, marked edema, abscesses, and severe inflammation were observed in the control group skin flaps (Fig. 4A). The skin flaps in the experimental group had normal epidermis and dermis; however, inflammatory cells and fibrosis were observed in the subcutaneous layer (Fig. 4B). Partial formation of granulation tissue and increased formation of capillary vessels were observed in the experimental group.
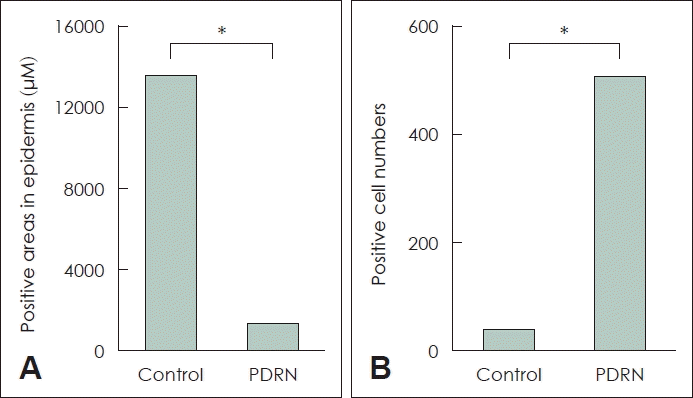
Immunohistochemistry (IHC) of IL-1β and VEGFα was performed to identify the reason for the PDRN group’s increased skin flap survival (Fig. 4C-F). The experimental group (1286 μm2/microscopy field at ×20 magnification) had significantly lower IL-1β expression than the control group (13620.33 μm2/microscopy field at ×20 magnification; p<0.05). The experimental group had significantly higher VEGFα expression than the control group (506/mm2 versus 32/mm2; p<0.05) (Fig. 5).
This study showed that subdermally injected PDRN enhances the survival of random skin flaps by increasing angiogenesis and reducing inflammation. The PDRN group had minimal histopathological changes during flap necrosis, lower IL-1β expression, and higher VEGFα expression in IHC staining. These findings suggest the potential therapeutic role of PDRN for random skin flap necrosis.
Necrosis of the distal part of the skin flap is the most significant limitation when using random skin flaps in reconstructive surgery. Several medications used to prevent distal necrosis have been reported. PDRN has anti-ischemic and anti-inflammatory effects and can be used as regenerative medicine [9]. PDRN is a deoxyribonucleotide mixture of 50-1500 kDa and is extracted from salmon trout or chum salmon semen DNA through purification and sterilization processes that removes proteins and peptides that can elicit immune responses. In vivo studies have revealed that the main mechanism of PDRN is the activation of the adenosine A2A receptor that plays a central role in regulating inflammation, ischemia, and angiogenesis [9].
Chung, et al. [11] have reported that intraperitoneally injected PDRN has protective effects for rat skin flap models. Lee, et al. [12] have demonstrated that PDRN has positive effects on skin flap survival after it is subdermally injected into rat skin flap models 2 days before and immediately after surgery. This study investigated the protective effects of a single, local injection of PDRN immediately after surgery. After PDRN injection, the VEGFα level was increased, and the IL-1β level was decreased compared to the levels in animals injected with PBS.
VEGF facilitates wound healing by promoting angiogenesis. It increases vascular permeability and degrades the extracellular matrix (ECM) to facilitate endothelial cell migration and proliferation and prevent endothelial cell apoptosis [13,14]. In ischemic tissues, the level of VEGF in endothelial cells increases, angiogenesis is promoted, and mediators are supplied through capillaries to promote healing [15]. Angiogenesis promoted by increased VEGF induces granulation tissue formation and maturation, elastic fiber formation, and rapid healing to protect the tissue from necrosis [16]. In this study, PDRN was locally injected into the skin flaps, and VEGF expression was analyzed using IHC 7 days later. Increased VEGF expression was observed after PDRN injection, especially around new vessels. Moreover, an increased number of VEGF-positive cells per microscopic field was noted in the mice injected with PDRN. These results suggest that PDRN increases VEGF expression during the conditions of skin necrosis to promote angiogenesis, thereby contributing to wound healing. PDRN is known to increase VEGF expression by binding adenosine receptors. Adenosine is a purine nucleoside containing adenine and ribose. Recent studies report that purinergic signaling can improve wound healing [17,18]. Future studies regarding the mechanism of purinergic signaling are warranted.
IL-1β is a central cytokine regulating inflammation and immune responses to infections. IL-1 consists of IL-1α and IL-1β, which are expressed by different genes [19]. IL-1β is a major pro-inflammatory interleukin. When IL-1β-driven inflammatory signals are overexpressed at a wound site, inflammatory cells stay at the site longer, and the level of matrix metalloproteinases that degrade the ECM increases, delaying wound healing [20]. Blocking IL-1β expression is considered an effective strategy to reduce inflammation and promote wound healing [21]. In this study, a decreased expression of IL-1β was observed in the experimental group 7 days postoperatively, suggesting that PDRN inhibited inflammatory reactions by reducing IL-1β expression, resulting in protection of the skin from necrosis.
In this study, an LSCI system was used to examine changes in blood flow within the skin flaps 7 days postoperatively. This imaging system analyzes changes in laser speckle patterns produced by blood flow to obtain quantitative and tangible data regarding the relative motion of blood flow. The gross anatomical findings were consistent with the LSCI findings. A higher perfusion index in the distal part of the flap was observed in the experimental group of mice. Additionally, the experimental group showed better blood flow preservation in the flap than the control group.
Preclinical studies have reported that PDRN has tissue regenerative and anti-ischemic properties. PDRN has been shown to restore damaged skin and promote wound healing by increased VEGF expression in diabetic mice [22]. Furthermore, PDRN improved circulation in an animal model of peripheral arterial occlusive disease through increased VEGF expression [23]. PDRN selectively acts on adenosine A2 receptors to promote VEGF secretion [24]. VEGF promotes cell differentiation to increase vessel dilation and capillary formation, increasing and restoring the nutrient supply and blood flow in tissues [25,26]. Combined with the results of this study, these previous findings suggest that PDRN may be effective in clinical settings for the restoration of damaged flaps and improved flap survival.
There are several challenges in applying these results to real-world clinical practice. There are various animal studies on drugs that enhance flap necrosis; however, few drugs are actually used in humans. Large-animal experiments and clinical trials must be carried out for clinical use, as this study was only applied to small animals. Additionally, when PDRN is injected into the human body, it is crucial to evaluate the appropriate injection dose, injection route, and injection timing. Although the results after 1 week were confirmed in this study, additional studies are required on the long-term efficacy and side effects of PDRN. Further, to confirm the exact mechanism of action of PDRN, studies on various biomarkers should be performed.
In conclusion, PDRN inhibits flap necrosis during the necrotic process. The results of this study suggest that PDRN promotes fibroblast activity and ECM accumulation by increasing the formation of new capillaries, re-epithelialization, and wound contraction speed at the wound site. Therefore, PDRN may be effective in treating skin flap wounds in clinical settings.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2017R1D1A1B04031319).
Notes
Author Contribution
Conceptualization: Jiye Kim, Jaemoon Yang, Ji-Hoon Kim. Data curation: Jinhyuck Im, Ji Yong Lee. Methodology: Minhee Ku, Jinhyuck Im, Ji Yong Lee. Software: Jinhyuck Im. Supervision: Yoon Woo Koh, Eun Chang Choi, Nam Suk Sim. Validation: Nam Suk Sim. Visualization: Minhee Ku, Ji-Hoon Kim. Writing—original draft: Jiye Kim. Writing—review & editing: Ji-Hoon Kim.
REFERENCES
1. Fujioka M. Surgical reconstruction of radiation injuries. Adv Wound Care (New Rochelle). 2014; 3(1):25–37.
2. Starkman SJ, Williams CT, Sherris DA. Flap basics I: Rotation and transposition flaps. Facial Plast Surg Clin North Am. 2017; 25(3):313–21.
3. Lucas JB. The physiology and biomechanics of skin flaps. Facial Plast Surg Clin North Am. 2017; 25(3):303–11.
4. Schultz TA, Cunningham K, Bailey JS. Basic flap design. Oral Maxillofac Surg Clin North Am. 2014; 26(3):277–303.
5. Bayati S, Russell RC, Roth AC. Stimulation of angiogenesis to improve the viability of prefabricated flaps. Plast Reconstr Surg. 1998; 101(5):1290–5.
6. Wu H, Chen H, Zheng Z, Li J, Ding J, Huang Z, et al. Trehalose promotes the survival of random-pattern skin flaps by TFEB mediated autophagy enhancement. Cell Death Dis. 2019; 10(7):483.
7. Zhou KL, Zhang YH, Lin DS, Tao XY, Xu HZ. Effects of calcitriol on random skin flap survival in rats. Sci Rep. 2016; 6:18945.
8. Lin J, Lin R, Li S, Wu H, Ding J, Xiang G, et al. Protective effects of resveratrol on random-pattern skin flap survival: An experimental study. Am J Transl Res. 2019; 11(1):379–92.
9. Squadrito F, Bitto A, Irrera N, Pizzino G, Pallio G, Minutoli L, et al. Pharmacological activity and clinical use of PDRN. Front Pharmacol. 2017; 8:224.
10. Im J, Kong TH, Choi JS, Seo YJ, Choi EC, Jung B, et al. Noninvasive postoperative monitoring of pedicled rat skin flap using laser speckle contrast imaging. Microvasc Res. 2020; 132:104050.
11. Chung KI, Kim HK, Kim WS, Bae TH. The effects of polydeoxyribonucleotide on the survival of random pattern skin flaps in rats. Arch Plast Surg. 2013; 40(3):181–6.
12. Lee DW, Hong HJ, Roh H, Lee WJ. The effect of polydeoxyribonucleotide on ischemic rat skin flap survival. Ann Plast Surg. 2015; 75(1):84–90.
13. Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998; 97(1):99–107.
14. Spyridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner JM, et al. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: Balance between growth and death signals. J Mol Cell Cardiol. 1997; 29(5):1321–30.
15. Klein SA, Bond SJ, Gupta SC, Yacoub OA, Anderson GL. Angiogenesis inhibitor TNP-470 inhibits murine cutaneous wound healing. J Surg Res. 1999; 82(2):268–74.
16. Ram M, Singh V, Kumawat S, Kumar D, Lingaraju MC, Uttam Singh T, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. Eur J Pharmacol. 2015; 764:9–21.
17. Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res. 2007; 85(1):130–41.
18. Burnstock G, Knight GE, Greig AV. Purinergic signaling in healthy and diseased skin. J Invest Dermatol. 2012; 132(3 Pt 1):526–46.
19. Dinarello CA. Biology of interleukin 1. FASEB J. 1998; 2(2):108–15.
20. Tan JL, Lash B, Karami R, Nayer B, Lu YZ, Piotto C, et al. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun Biol. 2021; 4(1):422.
21. Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013; 62(7):2579–87.
22. Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calò M, et al. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008; 16(2):208–17.
23. Bitto A, Polito F, Altavilla D, Minutoli L, Migliorato A, Squadrito F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J Vasc Surg. 2008; 48(5):1292–300.
24. Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, et al. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009; 7(4):313–21.
25. Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004; 164(6):1887–92.
26. Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009; 77(7):1117–24.
Fig. 1.
Photographs of mouse model flap design. A: Rectangular random skin flap on the dorsal side of the mouse. B: A caudally based flap elevation was performed and a silicone sheet was inserted under the skin flap.
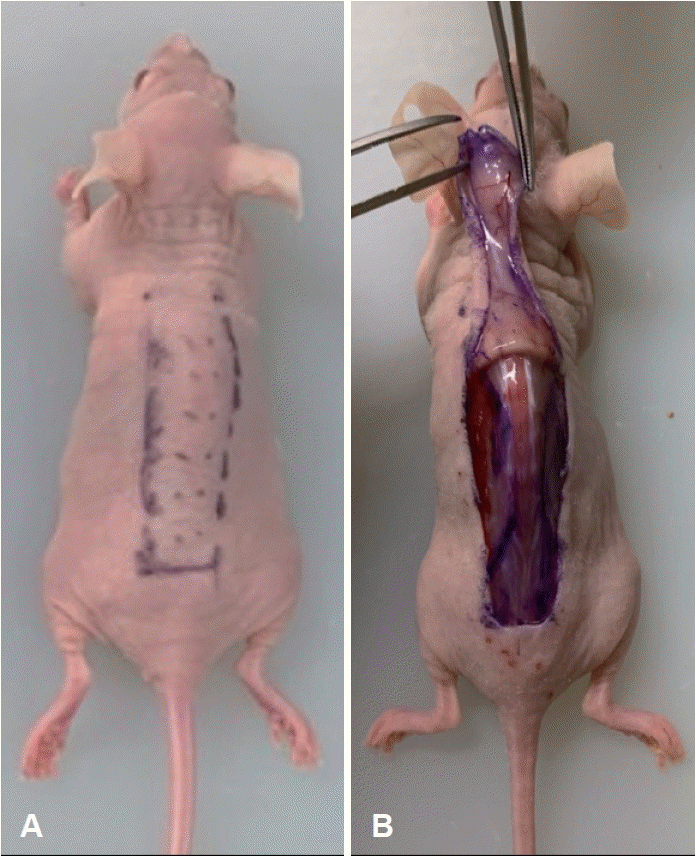
Fig. 2.
Images of skin flap on postoperative day 7. A: Photograph from the PDRN group. B: Laser speckle contrast image from the PDRN group. C: Inner surface of skin tissue from the PDRN group. D: Photograph from the PBS group. E: Laser speckle contrast image from the PBS group. F: Inner surface of skin tissue from the PBS group. PDRN, polydeoxyribonucleotide; PBS, pentobarbital sodium.
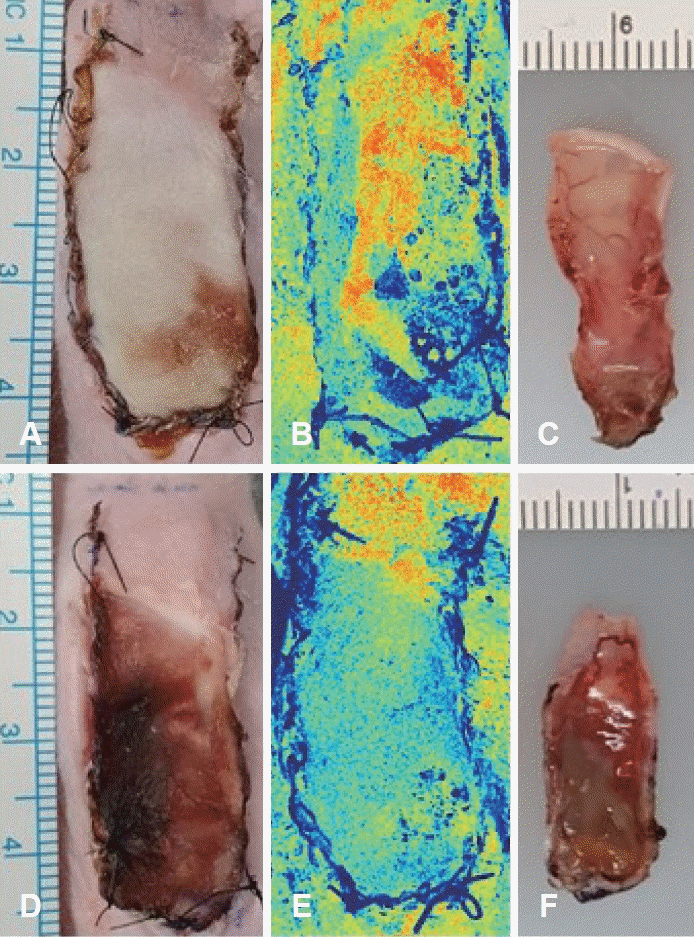
Fig. 3.
The laser speckle contrast imaging perfusion index ratio of the skin flap. *p<0.05. PDRN, polydeoxyribonucleotide (Perfusion index ratio=perfusion signal of distal part/perfusion signal of normal skin).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download