Abstract
Background and Objectives
The aim of this study was to identify the frequency of indicators of National Health Insurance (NHI) coverage for positive airway pressure (PAP) therapy and to investigate the changes in patients receiving coverage for PAP therapy after the alterations were made in the insurance benefit standards for mild obstructive sleep apnea (OSA).
Subjects and Method
We divided the mild OSA patients into two groups according to altered categorization in insurance benefit standards (Mild1: 5≤AHI<10; Mild2: 10≤AHI<15). Eight indicators related to the NHI coverage were identified: four symptoms, three complications, and the minimal blood oxygen saturation during polysomnography (min SpO2) of ≤85% during polysomnography. We also investigated the change in the number of patients receiving insurance benefits under the altered insurance benefit standards.
Results
Of the 233 OSA patients, 66, 57, and 110 patients were diagnosed as mild, moderate and severe OSA, respectively. For all of them, the most common indicator related to NHI coverage for PAP therapy was the minimum SpO2 of less than 85% during polysomnography, and the second most common indicator was daytime sleepiness. In the mild OSA group, however, daytime sleepiness was found to be the most common indicator, found in 46 (70%) patients, followed by 38 (58%) patients with min SpO2 of less than 85%. In this group, 59 (89.4%) would have been benefited before the change in the insurance benefit standards whereas 51 patients (77.3%) would now be benefited under the changed insurance benefit standards.
Obstructive sleep apnea (OSA) is caused by repeated narrowing or obstruction of the airway during sleep. It is a relatively common disease in otolaryngology, and patients present with symptoms related to blood-oxygen desaturation. Patients with OSA show many clinical symptoms, including habitual snoring, frequent arousal, daytime sleepiness, non-restorative sleep, cognitive decline, sleep apnea, and mood disorder. Frequent airway obstruction during sleep causes a decrease in blood oxygen saturation, and frequent waking occurs when the blood oxygen saturation is reduced. Frequent waking during sleep activates the sympathetic nervous system, leading to complications such as cardiovascular disease (CVD) [1,2].
There are several methods for managing OSA, including weight loss, intraoral devices, positive airway pressure (PAP) therapy, and surgery. PAP therapy helps maintain upper airway patency by acting as a pneumatic splint during sleep, reducing respiratory obstructive events such as daytime sleepiness, cognitive decline, hypertension, mood disorder, ischemic heart disease, and quality of life, and improves outcomes [3,4]. Therefore, since it was first introduced in the 1980s, PAP therapy has been a standard treatment for patients with OSA because it is non-invasive and effective even in patients with low surgical success [5].
In PAP therapy, adherence is important to obtain an appropriate treatment result, and economic factors have a significant influence on adherence. In particular, a reduction in financial burden plays an important role in increasing PAP device adherence [6,7]. The use of PAP therapy has been a benefit-support item for health insurance in Korea since July 2018. As a result, patient compliance, adherence, and use of PAP therapy increased [8,9]. Since July 2018, the Korean National Health Insurance (NHI) has been covering PAP therapy in patients with moderate-to-severe OSA (apnea hypopnea index [AHI] ≥15) and some complicated patients (lowest SpO2 <85% or lowest SpO2 ≥85% with insomnia, daytime sleepiness, cognitive disorder, mood disorder, hypertension, history of CVD or history of cerebrovascular disease) in mild OSA (5≤AHI<15). However, the salary standard for the use of PAP therapy in the mild OSA group has been strengthened since December 2020. After the insurance benefit standard was changed, patients with mild OSA were divided into two groups (Mild1: 5≤AHI<10, Mild2: 10≤AHI<15). The Mild1 group received insurance benefits in the presence of one or more of the following: hypertension, ischemic CVD, history of stroke or minimal blood oxygen saturation during polysomnography (min SpO2) less than 85%, while the Mild2 group received insurance benefits under same conditions previously mentioned.
In this study, the frequency of the symptoms and complications related to the insurance benefit of PAP therapy and the changes in the patients covered by NHI due to changes in the NHI benefit standards was investigated.
From March 2018 to November 2020, polysomnography (PSG) test results of patients who visited the Korea University Ansan Hospital ENT clinic for snoring and sleep apnea were retrospectively reviewed. Exclusion criteria were: 1) age under 18 years; 2) subjects who underwent PSG or a pulmonary function test at institutions other than the mentioned center; 3) unavailable or incomplete medical data; and 4) neuromuscular or craniofacial disorder. In total, 263 patients (224 male and 39 female) were included in this study. This study was reviewed and approved by the Institutional Ethics Committee of the hospital (2021AS0331).
A self-reported questionnaire was administered at the first visit to all patients. The questionnaire is about four medical histories related to OSA, such as hypertension, diabetes, history of ischemic heart disease, and history of stroke. In addition, there are approximately four symptoms and complications related to PAP therapy insurance benefits, such as insomnia, daytime sleepiness, cognitive decline, and mood disorder. Results of some physical examinations, such as the modified Mallampatti score, Friedman tonsil grade, and Muller maneuver, were also recorded.
The patients underwent a full-night in-laboratory PSG (Alice 6; Philips Respironics, Atlanta, GA, USA). The study was conducted using four electroencephalogram channels, two electrooculograms, one submental and two leg electromyograms, one modified lead II electrocardiography, two airflow sensors (one oronasal thermistor and one nasal pressure transducer), two respiratory effort belts (chest and abdomen), pulse oximeter, microphone, and body position sensor. A sleep technician observed the behavior of the subjects and monitored their sleep positions using an infrared camera in the room. The technician also manually interpreted all sleep study results according to the standard criteria described in the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events. Apnea was defined as the absence of airflow for a period lasting at least 10 s, and hypopnea was defined as at least 30% reduction in airflow with concomitant 4% or greater decrease in oxygen saturation. The apnea index was defined as the number of apneic episodes per hour of the total sleep time (TST), and the AHI was defined as the number of episodes of apnea and hypopnea per hour of the TST. The arousal index was defined as the number of arousals per hour of the TST. The subjects were divided into four groups based on the severity of OSA as follows: control (AHI<5), mild (5≤AHI<15), moderate (15≤AHI<30), and severe (AHI≥ 30). Patients with mild OSA were divided into two groups (Mild1=5≤AHI<10 and Mild2=10≤ AHI<15).
Data for a continuous variable of samples are presented as mean±standard deviation. The distribution of AHI data was verified for normal. The McNemar test was performed to determine whether the change in the number of patients eligible for insurance benefits according to the changed NHI benefit standards for the same entire patient group was statistically significant. According to the McNemar test, when p<0.05, there was a statistically significant difference in the change in the insurance benefit patient group according to changes in the NHI benefit standards. Statistical analysis was performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA).
Control groups were excluded from the analysis because this study included patients with OSA. Baseline characteristics of the patients are shown in Table 1, for both overall and AHI categories, and there were 233 patients (male:female = 205:28). Table 2 summarizes the number of indicators, such as symptoms, complications, or test results related to NHI coverage of PAP therapy, overall, and by group of AHI severity. The most common indicator among OSA patients was min SpO2 ≤85%, followed by daytime sleepiness. Table 3 represents the demographics of mild OSA patients according to comorbidities which related to NIH coverage.
Table 4 shows the characteristics of the subgroups in the mild group (Mild1 and Mild2). Table 5 is organized into the same indicators as those in Table 2 for the Mild1 and Mild2 groups. Unlike in the total OSA and Mild2 groups, daytime sleepiness was the most common indicator in the mild group and the Mild1 group.
Changes were applied to the patients before and after the NHI standards. There was no difference in the number of people receiving NHI benefits in the Mild2, moderate, and severe groups, because there was no standard change. Fig. 1 shows the change in the number of people receiving NHI benefits owing to changes in the NHI benefit standards in the mild and mild subgroups.
In OSA patients, 226 out of 233 patients were eligible for NHI coverage before changes in the NHI benefit standards; however, 218 out of 233 patients were eligible for NHI coverage after the change. In summary, after the NHI standard change, the insurance benefit rate for PAP therapy significantly decreased by 3.4% from 97.0% to 93.6% (p<0.05).
Before the NHI standard change, 30 out of 34 patients in Mild1 group and 29 out of 32 in Mild2 group were eligible for insurance benefits, and 59 out of 66 (89.4%) patients in mild group were eligible for insurance coverage. However, after changes in the NHI benefit standards, 22 out of 34 patients in Mild1 group were eligible for insurance benefits, and 51 out of 66 (77.3%) patients in mild group were eligible for insurance coverage. After the NHI standard changes, the insurance benefit rate for PAP therapy significantly decreased by 12.1%, from 89.4% to 77.3% in the mild OSA group (p<0.05) (Fig. 1).
The present study examined the frequency of the symptoms and complications related to PAP therapy insurance benefits. For the total OSA patient group, the most common indicators related to NHI coverage for PAP therapy were min SpO 2 less than 85% during PSG, and the second most common symptom was daytime sleepiness. However, in the mild group, the most common was daytime sleepiness, and the second most common was min SpO 2 less than 85% during PSG. Various studies reported that excessive daytime sleepiness was significantly correlated with various novel polysomnographic parameters such as obstruction duration, desaturation duration, desaturation severity, and obstructive severity. Moreover, when compared to AHI, excessive daytime sleepiness was more strongly correlated with these novel parameters [10]. Mild OSA patients with excessive daytime sleepiness experience significant burden of the disease than that of without excessive daytime sleepiness [11]. It is well known that PAP therapy significantly eliminates excessive daytime sleepiness, and this improves the quality of life of the patients [12]. Therefore, the treatment using PAP device in mild OSA patients with excessive sleepiness is also important. The change in the number of patients receiving benefits when health insurance standards before and after the change were applied to the same patient group was also investigated. When the changed NHI benefit standard was applied to the mild group, the number of beneficiaries decreased by 12.1%, from 59 out of 66 to 51, and for all OSA patients, a decrease of 3.4% from 226 out of 233 to 218 was observed.
If OSA is not treated, various symptoms and complications may occur, resulting in direct and indirect medical costs [13,14]. OSA is associated with many conditions, such as hypertension, ischemic heart disease, and stroke, and these directly incur medical costs. Several studies have reported that patients with sleep apnea have higher healthcare costs than those in a control group [15-17]. In addition, excessive daytime sleepiness, the main symptom of sleep apnea, reduces concentration and the ability to respond immediately to unexpected events, resulting in accidents that can cause physical damage or economic loss. Several studies have reported that the risk of traffic accidents in patients with sleep apnea is higher in the untreated group than in the treated group [18,19]. Therefore, when OSA is controlled, various direct and indirect medical costs can be reduced, resulting in socioeconomic benefits.
Treatment of OSA includes weight control, postural therapy during sleep, intraoral devices, PAP therapy, and surgery [20]. Weight control, sleep posture therapy, and intraoral devices are noninvasive and simple. However, considering that it is difficult to expect a complete cure of OSA only by weight loss and that its therapeutic effect appears relatively late, it is better to combine weight loss with a primary treatment such as PAP therapy rather than a single treatment, and its success rate is not high [21]. Posture therapy is a method of maintaining the supine position during sleep. Likewise, it can be used as a secondary or adjunctive treatment for patients with sleep apnea rather than as a single treatment [22]. The intraoral device is suitable for the treatment of simple snoring, and its effect of improving excessive sleepiness is equivalent to that of PAP therapy; however, in terms of the AHI reduction effect, it is not as effective as PAP therapy [23]. There are various surgical methods to improve OSA, and the effect varies depending on the surgical method. However, the effective surgical method is often invasive, and uvulopalatopharyngoplasty, a relatively simple procedure, does not reliably normalize the AHI in moderate-to-severe OSA. PAP therapy has the advantage of being non-invasive and having a higher treatment success rate than other treatments. PAP therapy is non-invasive, effectively relieves AHI in moderate and severe OSA, and is known as the gold standard for OSA treatment [24].
Cost is an important consideration in treatment selection. In Korea, a high percentage of patients gave up treatment for OSA due to the burden of a PSG examination costing 400000 to 800000 won and purchasing a positive-pressure ventilator of around 2 million won before applying for insurance benefits. However, in July 2018, support for PSG and positive-pressure ventilation for sleep apnea began. As a result, only 20% of the examination cost has to be paid, and PAP equipment can be used for about 15200 won to 25200 won per month as a lease rather than a purchase. According to Kim, et al. [25], the number of patients diagnosed with sleep apnea increased rapidly from 2010 to 2017, in 2018 and 2019. In 2019, compared to 2018, the number of patients who underwent PSG increased by four times, and the number of patients enrolled in PAP increased by 3.11 times. As such, if the cost of diagnosis and treatment is reduced through NHI benefits, the number of patients receiving diagnosis and treatment will increase.
This study retrospectively analyzed the medical records of 233 patients. This hospital is a tertiary hospital, and since relatively severe patients visit the hospital through the community, the proportion of patients with moderate to severe OSA is higher than that of the general population. The fact that the patient’s symptoms and complications were not summarized based on the diagnosis but based on the patient’s statements can also be considered a limitation of the study. In addition, statistical analysis was performed on the assumption that PAP therapy was used when the NHI benefit criteria were satisfied for all patient groups; however, there were some patients who did not want the treatment or decided to undertake weight control.
The strength of this study is that it can directly visualize the change in the number of patients receiving insurance benefits when the changed NHI benefits are applied in clinical practice. As a result, it will be helpful in predicting changes in health insurance finances caused by changes in policy implementation and in devising additional plans. In addition, effective treatment will be possible if the ranking of common symptoms and complications in patients with OSA and management of those that occupy a large proportion are intensively managed.
In conclusion, in the OSA patient group, the most common indicator related with insurance benefits of PAP therapy was min SpO2 <85% during PSG, followed by daytime sleepiness. In the mild OSA group, daytime sleepiness was the most frequent, followed by min SpO2 <85%. After the change in the NHI benefit standards for the PAP therapy, the proportion of patients receiving insurance benefits significantly decreased from 89.4% to 77.3% in the mild OSA group.
In July 2018, PSG and PAP therapy in patients with OSA was covered the NHI. Since then, PSG and PAP therapy have increased rapidly, resulting in an increase in the financial loss to the NHI Corporation. Therefore, since December 2020, reimbursement standards for PAP treatment have been strengthened, especially in patients with mild OSA with an AHI of 5-10. Even if there is excessive daytime sleepiness, insurance benefits will not be available if there are no complications. As a result, the number of people receiving benefits from PAP therapy has decreased, similar to the results in this study. However, it is necessary to provide NHI benefits, if sufficient funds are gradually secured, for patients with mild OSA with severe daytime sleepiness and an AHI of 5-10, since excessive daytime sleepiness may threaten patient safety and lead to social costs.
REFERENCES
1. Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990; 336(8710):261–4.
2. Seo MY, Lee JY, Hahn JY, Ryu G, Hong SD, Dhong HJ, et al. Association of obstructive sleep apnea with subclinical cardiovascular disease predicted by coronary artery calcium score in asymptomatic subjects. Am J Cardiol. 2017; 120(4):577–81.
3. Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011; 34(1):111–9.
4. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016; 375(10):919–31.
5. Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981; 1(8225):862–5.
6. Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. 2009; 32(4):545–52.
7. Bakker JP, O’Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: Effects of socioeconomic status, health literacy and self-efficacy. Sleep. 2011; 34(11):1595–603.
8. Yoon HE, Jeon CJ, Hwang J, Lee HW, Jeon JY. Improved adherence to positive airway pressure treatment after covering national health insurance in patient with obstructive sleep apnea: A tertiary sleep center review. J Sleep Med. 2021; 18(1):22–8.
9. Choi W, Bae M, Chung Y. The impact of national health insurance coverage on compliance with positive airway pressure therapy in patients with obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2022; 15(1):100–6.
10. Cielo CM, Tapia IE. Diving deeper: Rethinking AHI as the primary measure of OSA severity. J Clin Sleep Med. 2019; 15(8):1075–6.
11. Landzberg D, Bagai K. Prevalence of objective excessive daytime sleepiness in a cohort of patients with mild obstructive sleep apnea. Sleep Breath. In press. 2021.
12. Javaheri S, Javaheri S. Update on persistent excessive daytime sleepiness in OSA. Chest. 2020; 158(2):776–86.
13. Morsy NE, Farrag NS, Zaki NFW, Badawy AY, Abdelhafez SA, El-Gilany AH, et al. Obstructive sleep apnea: Personal, societal, public health, and legal implications. Rev Environ Health. 2019; 34(2):153–69.
14. Lee HW, Kang BW, Park SP. Obstructive sleep apnea syndrome and socioeconomic impact. J Korean Sleep Res Soc. 2007; 4(1):30–3.
15. Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999; 22(6):749–55.
16. Ronald J, Delaive K, Roos L, Manfreda J, Bahammam A, Kryger MH. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999; 22(2):225–9.
17. Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999; 22(6):740–7.
18. Findley LJ, Suratt PM. Serious motor vehicle crashes: The cost of untreated sleep apnoea. Thorax. 2001; 56(7):505.
19. George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001; 56(7):508–12.
20. Sunwoo JS, Yang KI. Overview of treatment for obstructive sleep apnea in adults. J Sleep Med. 2017; 14(1):1–9.
21. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 5(3):263–76.
22. Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006; 29(8):1031–5.
23. Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: An update for 2005. Sleep. 2006; 29(2):240–3.
24. Cao MT, Sternbach JM, Guilleminault C. Continuous positive airway pressure therapy in obstuctive sleep apnea: Benefits and alternatives. Expert Rev Respir Med. 2017; 11(4):259–72.
25. Kim M, Baek H, Lee SY. Trends of clinical practice for obstructive sleep apnea following the change in the national health insurance coverage. J Sleep Med. 2020; 17(2):122–7.
Fig. 1.
Change in the number of patients eligible for benefits when applying before and after the change in the National Health Insurance benefit standard for positive airway pressure therapy in the mild OSA group. OSA, obstructive sleep apnea.
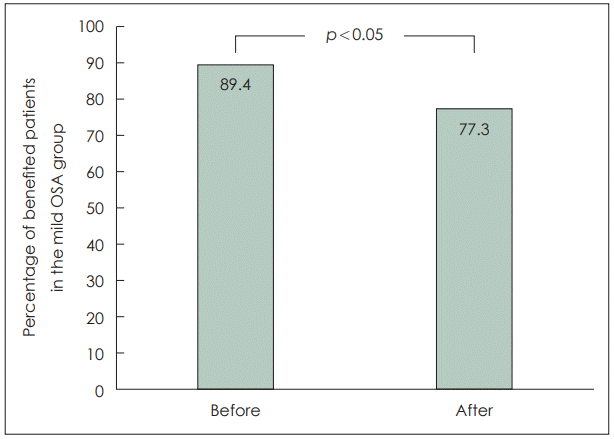
Table 1.
Patient demographics
Table 2.
Number of patients with symptoms, complications, or test results related to polysomnography insurance benefits by group according to overall and apnea hypopnea index severity
Table 3.
Demographics of mild obstructive sleep apnea patients according to comorbidities
Data are presented as mean±standard deviation. The p-value represents the difference for each factors according to presence of comorbidities. HTN, hypertension; CVD, cardiovascular disease; min SpO2, minimal blood oxygen saturation during polysomnography; BMI, body mass index; AHI, apnea hypopnea index
Table 4.
Demographics of mild obstructive sleep apnea patients and its subgroups
Table 5.
Number of patients with symptoms, complications, or test results related to polysomnography insurance benefits by the mild group and its subgroups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download