Abstract
Notes
Ethical statement
This study was approved by the institutional Review Board (IRB: ECASM-AIMS-2023-083). Written consent was obtained and signed by the legal guardians of the baby for full disclosure while maintaining strict confidentiality in respect to the patient medical information and images.
REFERENCES
Figure 1.
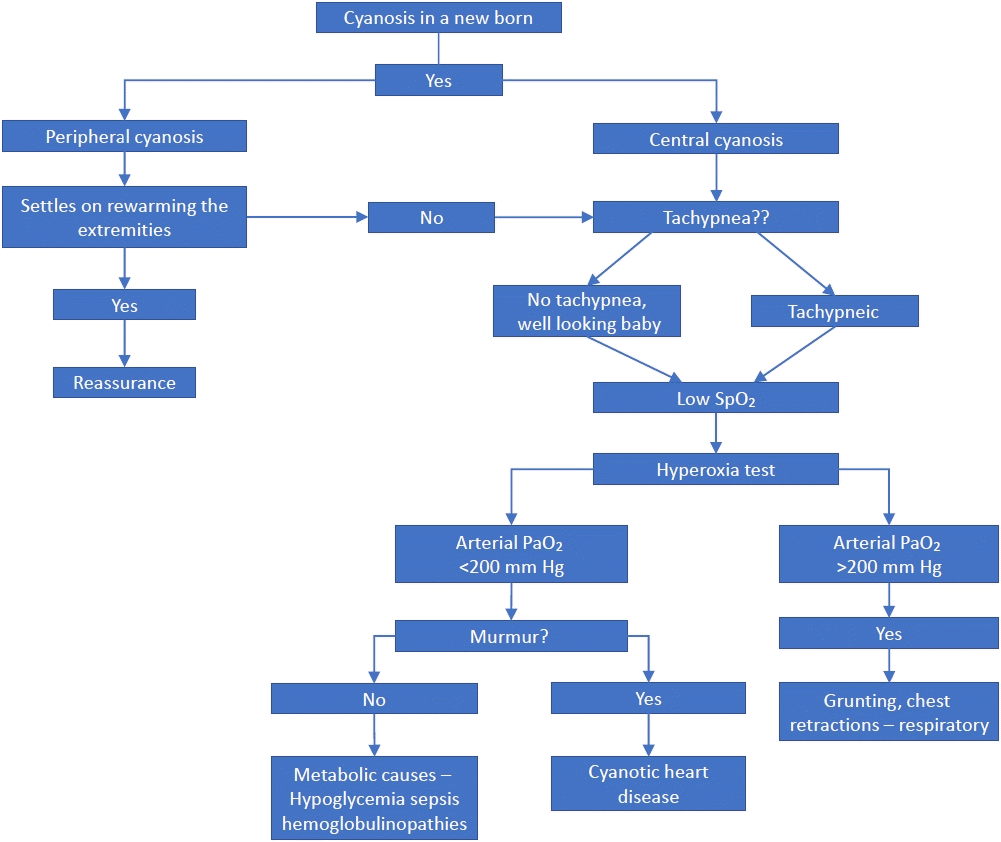
Table 1.
| Study | Treatment given | Result |
|---|---|---|
| Chandran et al. (2022) [9] | Hyperoxia test negative; MetHb levels 7.6% | p.His63Tyr |
| Weaned off to room air by 16 hours | ||
| Intravenous single dose methylene blue, then oral ascorbic acid 300 mg once daily | ||
| Crowley et al. (2011) [10] | SpO2 30%–50%, and PaO2 was 107 mm Hg. | p.Val67Met |
| Cyanosis resolved by 3 months, MetHb level was normal. | ||
| Bento et al. (2013) [11] | Hyperoxia tests was negative. | p.Leu28Met |
| CPAP ventilation and improved by day 6 | ||
| MetHb level of 16% | ||
| AlonsoOjembarrena et al. (2016) [12] | Hyperoxia tests negative | p.His63Tyr |
| Intubated and PGE1 infusion | ||
| MetHb 12.3%, weaned off to room air by day 4 | ||
| Yuan et al. (2020) [13] | Hyperoxia tests negative | p.Cyt190Thy |
| MetHb fluctuation between 2.6% and 5.7% | ||
| Assisted ventilation for 3 days and then hood oxygen | ||
| Vitamin C and vitamin B2 for 2 weeks | ||
| Hooven et al. (2016) [15] | Intubated at birth and extubated to nasal CPAP on day 2 of life | p.His93Tyr |
| MetHb levels were normal. | ||
| Discharged at 1 month of life with supplemental oxygen via nasal cannula | ||
| Ghotra et al. (2016) [16] | MetHb level of 1.7% | p.His92Tyr |
| Discharged on 200 cc of 100% FiO2 with SpO2 at discharge was 85%–87%. | ||
| Cyanosis resolved by 6 |
p.His63Tyr, p.His93Tyr, p.His92Tyr: Substitution of histidine (His) by tyrosine (Tyr) at amino acid 63, 93, and 92, respectively; p.Leu28Met, p.Leu29Met: Substitution of leucine (Leu) by methinone (Met) at amino acid 28 and 29, respectively; p.Val67Met: Substitution of valine (Val) by methionine (Met) at amino acid 67.
Abbreviations: MetHb, methemoglobin; SpO2, saturation of partial pressure oxygen; PaO2, arterial oxygen pressure; CPAP, continuous positive airway pressure; PGE1, prostaglandin E1; FiO2, fraction of inspired oxygen.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download