1. World Health Organization. Report of a WHO consultation: definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization, Department of Noncommunicable Disease Surveillance;1999.
2. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010; 1:212–28.
3. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020; 11:165–223.
4. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018; 61:2461–98.
5. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021; 45:461–81.
6. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S144–74.
7. Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021; 396:2019–82.
8. Ebrahim S, Smith GD. Systematic review of randomised controlled trials of multiple risk factor interventions for preventing coronary heart disease. BMJ. 1997; 314:1666–74.
9. Agewall S, Wikstrand J, Samuelsson O, Persson B, Andersson OK, Fagerberg B. The efficacy of multiple risk factor intervention in treated hypertensive men during long-term follow up. Risk Factor Intervention Study Group. J Intern Med. 1994; 236:651–9.
10. Kempler P. Learning from large cardiovascular clinical trials: classical cardiovascular risk factors. Diabetes Res Clin Pract. 2005; 68 Suppl 1:S43–7.
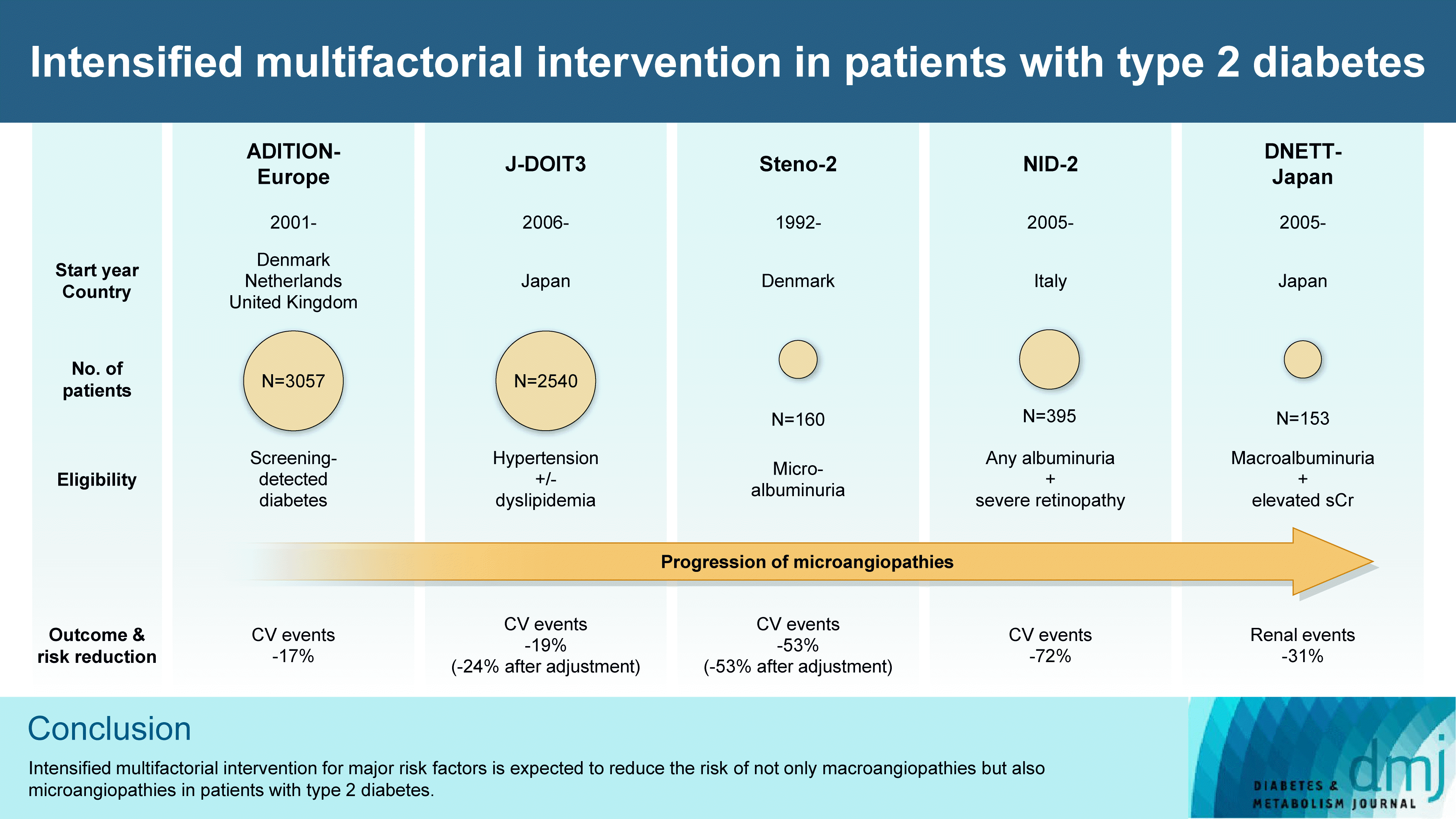
11. Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999; 353:617–22.
12. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003; 348:383–93.
13. Tanner M. In screen-detected type 2 diabetes, intensive therapy did not differ from usual care for CV events at 10 years. Ann Intern Med. 2020; 172:JC41.
14. Yagyu H, Shimano H. Treatment of diabetes mellitus has borne much fruit in the prevention of cardiovascular disease. J Diabetes Investig. 2022; 13:1472–88.
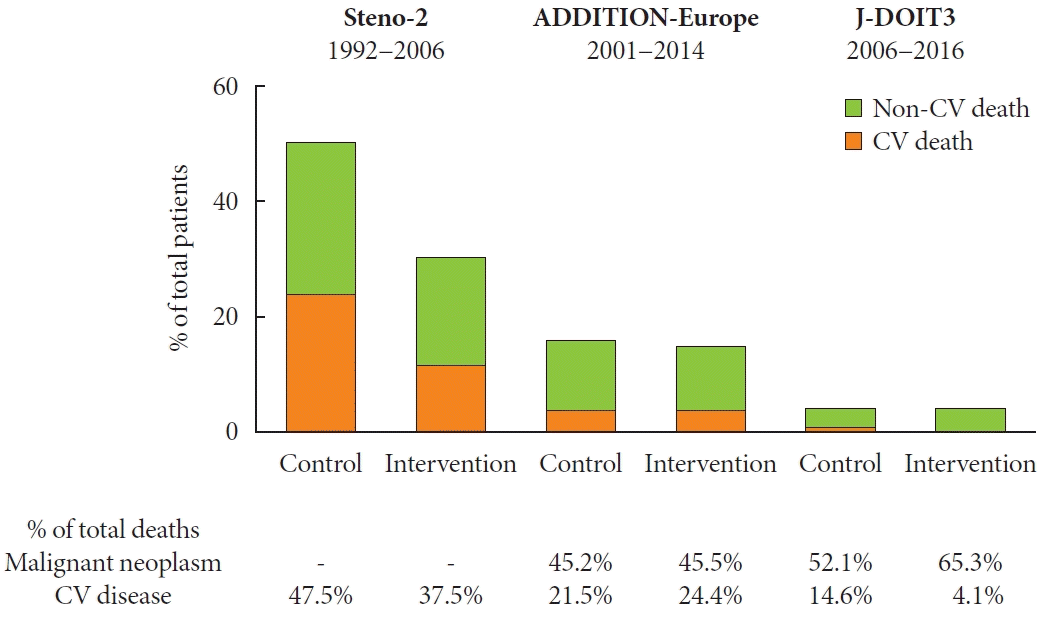
15. Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011; 378:156–67.
16. Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (JDOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017; 5:951–64.
17. Sasso FC, Pafundi PC, Simeon V, De Nicola L, Chiodini P, Galiero R, et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc Diabetol. 2021; 20:145.
18. Shikata K, Haneda M, Ninomiya T, Koya D, Suzuki Y, Suzuki D, et al. Randomized trial of an intensified, multifactorial intervention in patients with advanced-stage diabetic kidney disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan). J Diabetes Investig. 2021; 12:207–16.
19. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–91.
20. Gaede P, Pedersen O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes. 2004; 53 Suppl 3:S39–47.
21. Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001; 101:671–9.
22. Gaede P, Valentine WJ, Palmer AJ, Tucker DM, Lammert M, Parving HH, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care. 2008; 31:1510–5.
23. Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016; 59:2298–307.
24. Gaede P, Oellgaard J, Kruuse C, Rossing P, Parving HH, Pedersen O. Beneficial impact of intensified multifactorial intervention on risk of stroke: outcome of 21 years of follow-up in the randomised Steno-2 Study. Diabetologia. 2019; 62:1575–80.
25. Oellgaard J, Gaede P, Rossing P, Rorth R, Kober L, Parving HH, et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia. 2018; 61:1724–33.
26. Oellgaard J, Gaede P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 2017; 91:982–8.
27. Gaede J, Oellgaard J, Ibsen R, Gaede P, Nortoft E, Parving HH, et al. A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study. Diabetologia. 2019; 62:147–55.
28. Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000; 24 Suppl 3:S6–11.
29. Janssen PG, Gorter KJ, Stolk RP, Rutten GE. Randomised controlled trial of intensive multifactorial treatment for cardiovascular risk in patients with screen-detected type 2 diabetes: 1-year data from the ADDITION Netherlands study. Br J Gen Pract. 2009; 59:43–8.
30. Echouffo-Tcheugui JB, Simmons RK, Williams KM, Barling RS, Prevost AT, Kinmonth AL, et al. The ADDITION-Cambridge trial protocol: a cluster: randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health. 2009; 9:136.
31. Webb DR, Khunti K, Srinivasan B, Gray LJ, Taub N, Campbell S, et al. Rationale and design of the ADDITION-Leicester study, a systematic screening programme and randomised controlled trial of multi-factorial cardiovascular risk intervention in people with type 2 diabetes mellitus detected by screening. Trials. 2010; 11:16.
32. Sandbæk A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care. 2014; 37:2015–23.
33. Griffin SJ, Rutten GE, Khunti K, Witte DR, Lauritzen T, Sharp SJ, et al. Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster-randomised trial. Lancet Diabetes Endocrinol. 2019; 7:925–37.
34. Preiss D, Sattar N. The case for diabetes screening: ADDITION-Europe. Lancet. 2011; 378:106–8.
35. Simmons RK, Sharp SJ, Sandbaek A, Borch-Johnsen K, Davies MJ, Khunti K, et al. Does early intensive multifactorial treatment reduce total cardiovascular burden in individuals with screen-detected diabetes?: findings from the ADDITION-Europe cluster-randomized trial. Diabet Med. 2012; 29:e409–16.
36. Johansen NB, Charles M, Vistisen D, Rasmussen SS, Wiinberg N, Borch-Johnsen K, et al. Effect of intensive multifactorial treatment compared with routine care on aortic stiffness and central blood pressure among individuals with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2012; 35:2207–14.
37. Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screendetected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011; 34:2244–9.
38. Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, et al. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013; 56:101–8.
39. Koekkoek PS, Ruis C, van den Donk M, Biessels GJ, Gorter KJ, Kappelle LJ, et al. Intensive multifactorial treatment and cognitive functioning in screen-detected type 2 diabetes: the ADDITION-Netherlands study: a cluster-randomized trial. J Neurol Sci. 2012; 314:71–7.
40. Tao L, Wilson EC, Wareham NJ, Sandbaek A, Rutten GE, Lauritzen T, et al. Cost-effectiveness of intensive multifactorial treatment compared with routine care for individuals with screendetected type 2 diabetes: analysis of the ADDITION-UK cluster-randomized controlled trial. Diabet Med. 2015; 32:907–19.
41. Thoolen BJ, de Ridder DT, Bensing JM, Gorter KJ, Rutten GE. Psychological outcomes of patients with screen-detected type 2 diabetes: the influence of time since diagnosis and treatment intensity. Diabetes Care. 2006; 29:2257–62.
42. van den Donk M, Gorter KJ, Rutten GE. No negative effects of a multi-factorial, intensified treatment on self-reported health status, treatment satisfaction, and diabetes-related distress in screen-detected type 2 diabetes patients: the ADDITIONNetherlands study. Qual Life Res. 2010; 19:509–13.
43. Van den Donk M, Griffin SJ, Stellato RK, Simmons RK, Sandbaek A, Lauritzen T, et al. Effect of early intensive multifactorial therapy compared with routine care on self-reported health status, general well-being, diabetes-specific quality of life and treatment satisfaction in screen-detected type 2 diabetes mellitus patients (ADDITION-Europe): a cluster-randomised trial. Diabetologia. 2013; 56:2367–77.
44. Kuznetsov L, Griffin SJ, Davies MJ, Lauritzen T, Khunti K, Rutten GE, et al. Diabetes-specific quality of life but not health status is independently associated with glycaemic control among patients with type 2 diabetes: a cross-sectional analysis of the ADDITION-Europe trial cohort. Diabetes Res Clin Pract. 2014; 104:281–7.
45. Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, Sargeant LA, Williams KM, Prevost AT, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. Lancet. 2012; 380:1741–8.
46. Dalsgaard EM, Sandbaek A, Griffin SJ, Rutten GE, Khunti K, Davies MJ, et al. Patient-reported outcomes after 10-year follow-up of intensive, multifactorial treatment in individuals with screen-detected type 2 diabetes: the ADDITION-Europe trial. Diabet Med. 2020; 37:1509–18.
47. Ueki K, Sasako T, Kato M, Okazaki Y, Okahata S, Katsuyama H, et al. Design of and rationale for the Japan Diabetes Optimal Integrated Treatment study for 3 major risk factors of cardiovascular diseases (J-DOIT3): a multicenter, open-label, randomized, parallel-group trial. BMJ Open Diabetes Res Care. 2016; 4:e000123.
48. Yazaki Y, Kadowaki T. Combating diabetes and obesity in Japan. Nat Med. 2006; 12:73–4.
49. Sakane N, Kotani K, Takahashi K, Sano Y, Tsuzaki K, Okazaki K, et al. Effects of telephone-delivered lifestyle support on the development of diabetes in participants at high risk of type 2 diabetes: J-DOIT1, a pragmatic cluster randomised trial. BMJ Open. 2015; 5:e007316.
50. Hayashino Y, Suzuki H, Yamazaki K, Goto A, Izumi K, Noda M. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: the Japan Diabetes Outcome Intervention Trial-2 (J-DOIT2). Diabet Med. 2016; 33:599–608.
51. Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021; 99:256–66.
52. Sasako T, Ueki K, Miyake K, Okazaki Y, Takeuchi Y, Ohashi Y, et al. Effect of a multifactorial intervention on fracture in patients with type 2 diabetes: subanalysis of the J-DOIT3 Study. J Clin Endocrinol Metab. 2021; 106:e2116–28.
53. Sasso FC, De Nicola L, Carbonara O, Nasti R, Minutolo R, Salvatore T, et al. Cardiovascular risk factors and disease management in type 2 diabetic patients with diabetic nephropathy. Diabetes Care. 2006; 29:498–503.
54. Sasso FC, Chiodini P, Carbonara O, De Nicola L, Conte G, Salvatore T, et al. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate: the NID-2 Prospective Cohort Study. Nephrol Dial Transplant. 2012; 27:2269–74.
55. Sasso FC, Marfella R, Pagano A, Porta G, Signoriello G, Lascar N, et al. Lack of effect of aspirin in primary CV prevention in type 2 diabetic patients with nephropathy: results from 8 years follow-up of NID-2 study. Acta Diabetol. 2015; 52:239–47.
56. Sasso FC, Lascar N, Ascione A, Carbonara O, De Nicola L, Minutolo R, et al. Moderate-intensity statin therapy seems ineffective in primary cardiovascular prevention in patients with type 2 diabetes complicated by nephropathy: a multicenter prospective 8 years follow up study. Cardiovasc Diabetol. 2016; 15:147.
57. Shikata K, Haneda M, Koya D, Suzuki Y, Tomino Y, Yamada K, et al. Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan): rationale and study design. Diabetes Res Clin Pract. 2010; 87:228–32.
58. Crasto W, Jarvis J, Khunti K, Skinner TC, Gray LJ, Brela J, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: the Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Res Clin Pract. 2011; 93:328–36.
59. Crasto W, Morrison AE, Gray LJ, John E, Jarvis J, Brela J, et al. The Microalbuminuria Education Medication and Optimisation (MEMO) study: 4 years follow-up of multifactorial intervention in high-risk individuals with type 2 diabetes. Diabet Med. 2020; 37:286–97.
60. Zhao XH, Xu ZR, Zhang Q, Gu HF, Yang YM. Effect of intensive multifactorial treatment on the intima-media thickness of large arteries in patients with new-onset type 2 diabetes mellitus. J Zhejiang Univ Sci B. 2012; 13:378–85.
61. Yang Y, Yao JJ, Du JL, Bai R, Sun LP, Sun GH, et al. Primary prevention of macroangiopathy in patients with short-duration type 2 diabetes by intensified multifactorial intervention: seven-year follow-up of diabetes complications in Chinese. Diabetes Care. 2013; 36:978–84.
62. Shi C, Men L, Yu C, Yao J, Bai R, Yang Y, et al. Atherosclerosis associated with dynamic inflammation changes after multifactorial intervention in short-duration type 2 diabetes: a randomized, controlled, 10-year follow-up trial. J Diabetes Complications. 2017; 31:1286–92.
63. Tripolt NJ, Narath SH, Eder M, Pieber TR, Wascher TC, Sourij H. Multiple risk factor intervention reduces carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2014; 13:95.
64. Shi C, Fang X, Yang Y, Bai R, Yu S, Sun G, et al. Intensive multifactorial intervention improved renal impairment in short-duration type 2 diabetes: a randomized, controlled, 7-year followup trial. J Diabetes Complications. 2020; 34:107468.
65. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care. 2009; 32:977–82.
66. Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, et al. Long-term multiple risk factor interventions in Japanese elderly diabetic patients: the Japanese Elderly Diabetes Intervention Trial: study design, baseline characteristics and effects of intervention. Geriatr Gerontol Int. 2012; 12 Suppl 1:7–17.
67. Solomon CG. Reducing cardiovascular risk in type 2 diabetes. N Engl J Med. 2003; 348:457–9.
68. Sasako T, Kadowaki T, Ueki K. ADDITION-Europe: the first decade and beyond. Lancet Diabetes Endocrinol. 2019; 7:891–3.
69. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–53.
70. Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia. 2010; 53:419–28.
71. Chan JCN. How can we optimise diabetes care in real-world practice? Lancet Diabetes Endocrinol. 2017; 5:927–9.
72. Araki E, Senokuchi T, Furukawa N. Impacts of tight multifactorial intervention in patients with type 2 diabetes: implications from the Japan Diabetes Outcome Intervention Trial 3. J Diabetes Investig. 2018; 9:1022–4.
73. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–89.
74. Hayward RA, Reaven PD, Emanuele NV; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015; 373:978.
75. Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, et al. Intensive glucose control in patients with type 2 diabetes: 15-year follow-up. N Engl J Med. 2019; 380:2215–24.
76. Sasako T, Yamauchi T. Addressing screams for evidence on renoprotection by GLP-1 receptor agonists. Kidney Int. 2022; 101:222–4.
77. Sasako T, Yamauchi T. Clinical trials in participants with type 2 diabetes undertaken in Japan. J Jpn Diabetes Soc. 2022; 65:518–21.
78. Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ. 2016; 353:i2200.
79. Neuen BL, Young T, Heerspink HJ, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019; 7:845–54.
80. Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020; 8:192–205.
81. Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018; 391:2430–40.
82. Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021; 9:165–73.
83. Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001- 2010: report of Committee on Causes of Death in Diabetes Mellitus. Diabetol Int. 2017; 8:117–36.
84. Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis. 1998; 137 Suppl:S65–73.
85. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009; 373:1765–72.
86. International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019; 7:385–96.
87. Roussel R, Steg PG, Mohammedi K, Marre M, Potier L. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: a perspective on glucose-lowering interventions. Diabetes Obes Metab. 2018; 20:238–44.
88. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes: state-of-the-art. Mol Metab. 2021; 46:101102.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download