Abstract
Objective
To establish a new treatment option for cervico-isthmic pregnancy (CIP) other than termination by maintaining pregnancy from diagnosis to delivery.
Methods
This retrospective observational study included women diagnosed with CIP at Dongsan Medical Center, Daegu, Korea, from January 2014 to December 2019. Eight patients were diagnosed with CIP using transvaginal ultrasound and met the following inclusion criteria: (1) preserved and closed cervical canal; and (2) more than half of the uterine cavity above the sac was not involved in sac implantation. Five of the eight mothers decided to maintain their pregnancy after an adequate explanation of the possible risks. The same sonographer assessed fetal and maternal status every 1–2 weeks. Intra- and postoperative indicators, delivery information, and neonatal outcomes were also recorded.
Results
The mean patient age was 36 years. In all cases, placenta accreta spectrum and placenta previa were detected using preoperative ultrasonography. A hysterectomy was performed in three cases, and all patients required intensive care unit (ICU) care. The mean operative time was 156 minutes. The rate of postpartum hemorrhage was 40%. Four viable fetuses were delivered. Birth preceding 34 weeks occurred in one patient, who required neonatal ICU hospitalization for 19 days.
Conclusion
Conservative treatment with careful diagnosis, management, and sufficient consultation could be an alternative treatment option in women with CIP, particularly older mothers, those with subfertility, and those who expect to have limited future opportunities for a successful pregnancy. Therefore, CIP should be treated as a separate disease entity.
Go to : 
Cervico-isthmic pregnancy (CIP) is a rare and potentially life-threatening ectopic pregnancy in which the fertilized egg implants in the isthmus between the histological and anatomical internal os. The internal cervical os is defined histologically as the transition area from the endocervical mucosa to the isthmic mucosa and anatomically as the transition area between the isthmus and uterine corpus [1–3].
Gestational products in CIP extend to the lower uterine segment; therefore, they can grow until the fetus is viable. Nevertheless, this can cause adverse events during early pregnancy, such as threatened abortion, spontaneous abortion, and uterine rupture, and tremendous complications, such as placenta previa, placenta accreta spectrum, postpartum hemorrhage requiring hysterectomy, intensive care unit (ICU) care, and even death. Due to potentially fatal complications, the diagnosis of CIP is typically followed by early termination of the pregnancy by methods including methotrexate (MTX) injection or dilation and curettage (D&C) [3]. Therefore, there are many cases in which clinicians do not distinguish CIP as a separate disorder or entity. Furthermore, it is difficult to accurately diagnose and distinguish CIP from cesarean section scar pregnancy (CSP) or cervical pregnancy (CP).
Some case reports have described successful management that resulted in term or near-term delivery. Here we identified 10 cases in the literature from the last 2 to 3 decades, including cases diagnosed at 24 weeks and others diagnosed during labor [2–10]. Here we present five cases of confirmed CIP that were closely monitored from diagnosis to delivery. We also discuss the outcomes of patient-tailored counseling provided at our center to women intending to maintain pregnancy. This could be a useful guide for clinicians treating women diagnosed with CIP who have limited opportunities for a successful pregnancy.
Go to : 
We retrospectively analyzed the data of patients with suspected CP, CSP, or CIP who were referred by local medical centers to Dongsan Medical Center, Daegu, Korea, from January 2014 to December 2019. This study was approved by a certified Institutional Review Board (DSMC 2020-12-008-005).
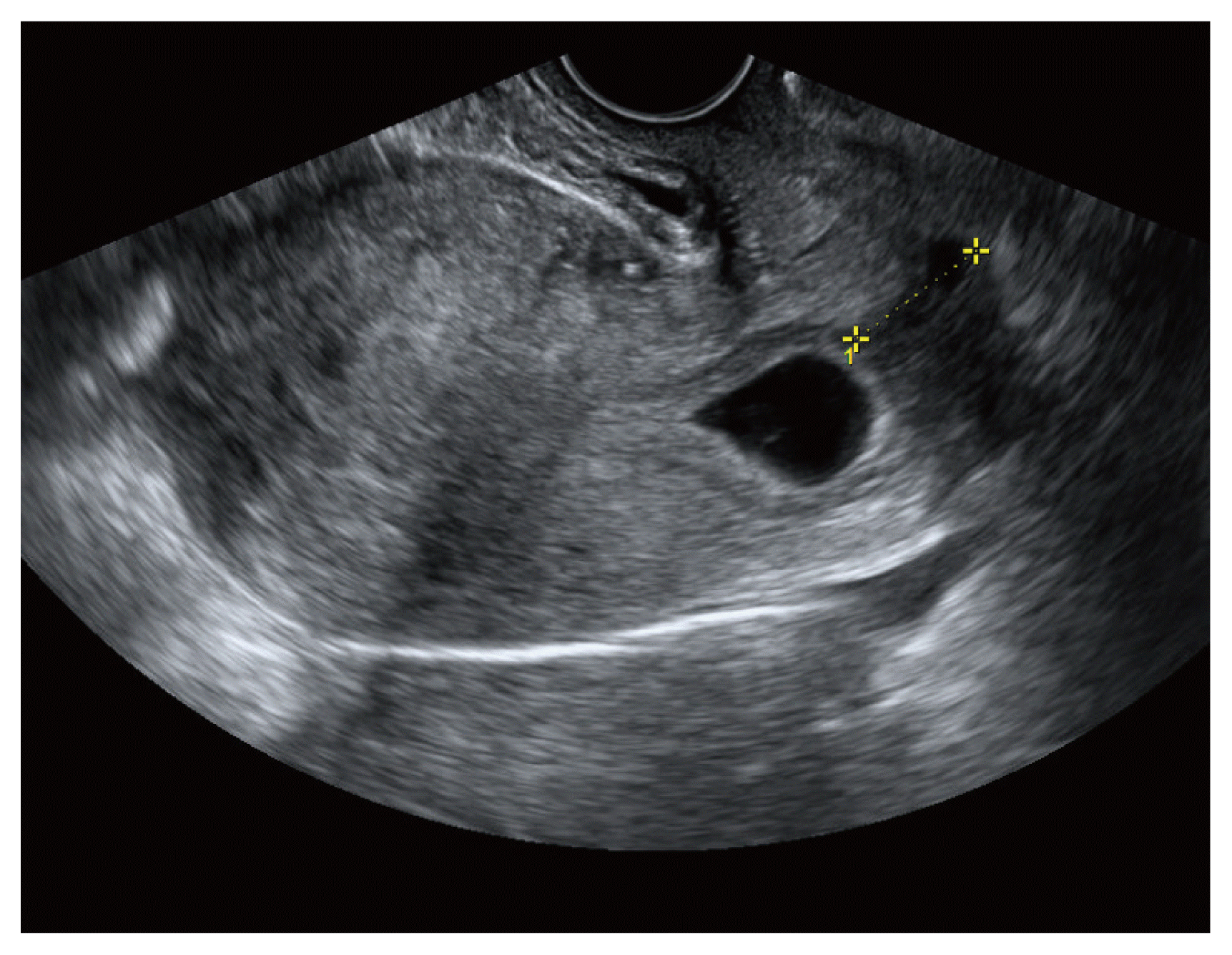
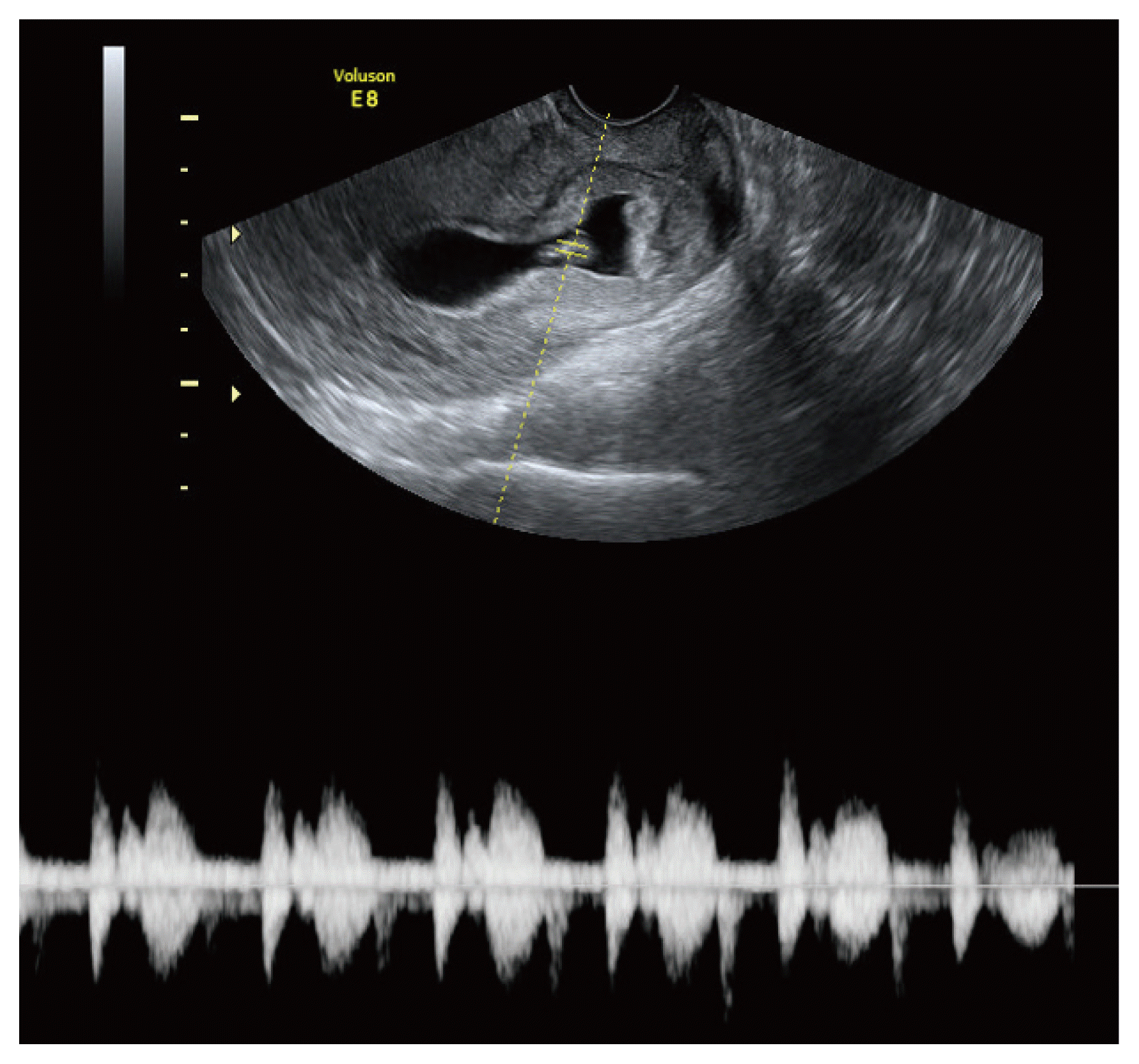
The CIP diagnosis was made using transvaginal ultrasonography based on two criteria: (1) preserved and closed cervical canal; and (2) more than half of the uterine cavity above the sac not involved in sac implantation (Fig. 1) [11,12]. It is particularly difficult to distinguish CIP from CP and CSP. Therefore, here we focused on the extension of the gestational sac (G-sac) based on gestational age as the distinguishing factor (Fig. 2). We followed up each patient with ultrasonography weekly, observed changes in the G-sac and cervical canal, and excluded cases of spontaneous abortion. All enrolled patients were examined by the same sonographer.
At the time of the CIP diagnosis, we provided each patient with comprehensive information regarding their current diagnosis, treatment, and possible adverse events during early pregnancy. We stressed the need for close follow-up because of the possibility of spontaneous abortion, abnormal growth of the G-sac, and vaginal bleeding during early pregnancy. We also informed patients that termination was not the only treatment option for CIP and that it was possible to maintain the pregnancy if consent was provided based on the patient’s wishes. All patients decided to maintain or terminate the pregnancy during the first or second center visit. We carefully counseled patients regarding their concerns and anxiety and encouraged them to take adequate time to make an informed decision but highlighted that their decision had to be made no later than 9 weeks’ gestation.
Furthermore, we explained the potential pregnancy-related complications, including vaginal bleeding due to placenta previa or uterine rupture that could lead to preterm birth; and the prognosis and risks associated with this type of pregnancy, including placenta accreta spectrum, postpartum hemorrhage followed by cesarean section or hysterectomy, ICU care, and even death.
Of the eight patients diagnosed with CIP, we included five who desired to maintain their pregnancy after discussing the potential severe complications and expected outcomes of the pregnancy. They visited our center for antepartum check-ups every 2 weeks from the second trimester or whenever they experienced contraction sensations, abdominal discomfort, or vaginal bleeding. We also provided the option to terminate the process at any time before 22 weeks of gestation. All deliveries were performed by cesarean section with adequate preparation for events, such as massive transfusion, angioembolization, hysterectomy, and ICU admission. Neonates were examined and monitored by a pediatrician immediately following delivery.
Go to : 
The basic characteristics of patients with CIP are described below. The mean patient age was 36 years (range, 35–37). All patients but case C had a history of cesarean section and had experienced one or two artificial abortions. Case C experienced a 3-year period of infertility and underwent artificial reproductive therapy for pregnancy. The reason for hospitalization during pregnancy was vaginal bleeding in cases A, B, and C, who underwent 1, 3, and 2 hospitalizations, with a total of 6, 8, and 18 hospitalization days until delivery, respectively. In case E, the pregnancy was maintained through outpatient follow-up without hospitalization until elective cesarean section (Table 1).
Basic characteristics of patients with cervico-isthmic pregnancy
In all cases, placenta previa and placenta accreta spectra were detected using preoperative ultrasonography; thus, the rate of placenta previa was 100%. Hysterectomy was performed in cases A, B, and D. All the patients required ICU care. The mean operative time was 156 minutes (range, 100–210), which was longer than the average time for cesarean sections. The mean estimated blood loss was 910 mL (range, 500–1,500) and the rate of postpartum hemorrhage was 40%. Transfusion was performed before and after surgery, and postoperative blood tests revealed no disseminated intravascular coagulation. The postoperative hemoglobin level exceeded 9 mg/dL in all cases. Case D required transfusion most urgently; this patient received 12 packs of red blood cells, 11 packs of fresh frozen plasma, and 14 packs of platelets. In all five cases, postoperative pathologic biopsy confirmed placenta accreta spectrum (Table 2).
Markers during surgery and postoperative care in cervico-isthmic pregnancies
Moreover, in one case (case D), the mother sought to terminate the pregnancy on day 1 at 19 weeks of gestation, followed by hysterectomy after angioembolization. Viable fetuses were delivered in all other cases. In case A, during hospitalization for vaginal bleeding, an emergency cesarean section was performed due to preterm labor at 31 weeks and 4 days, and the neonate weighed 1,920 g at delivery with 1- and 5-minute Apgar scores of 6 and 8, respectively. Case B also underwent an emergency cesarean section at 35 weeks an 1 day under similar circumstances and for the same reasons as case A; the neonate in case B weighed 2,570 g at delivery, with 1- and 5-minute Apgar scores of 5 and 7, respectively. Case C also underwent an emergency cesarean section due to preterm premature rupture of the membranes; the neonate weighed 2,150 g with 1- and 5-mintue Apgar scores of 7 and 9, respectively. Case E was delivered via elective cesarean section. Except for case A, none of the patients required neonatal ICU hospitalization (Table 3).
Neonatal outcomes in cases of cervico-isthmic pregnancies
Go to : 
In 2013, the Tsai group introduced the concept of low-lying-implantation ectopic pregnancy (LLIEP), which refers to cases where implantation occurs near the cervix and has been clearly classified into CP, CSP, and CIP [13].The overall characteristics, diagnosis, and treatment were analyzed in 42 patients with LLIEP. CIP was approached as a diagnosable exclusion entity when CP, CSP, and abortion were excluded. Until then, “cervico-isthmic pregnancy” was used as an umbrella term, but CIP was not diagnosed separately from CP and CSP because of the absence of appropriate diagnostic criteria. As CIP is a form of ectopic pregnancy, termination is the treatment of choice. This is highlighted in previous literature on CIP. Most studies presented the effects of each termination method, that is, MTX injection, D&C, or angioembolization, as treatments for CIP. The patients in all case reports describing conservative management and subsequent successful delivery were incidentally diagnosed after 20 weeks of gestation [11]. No study has reported conservative management starting at diagnosis during early pregnancy as a treatment option for CIP. In the 2013 study by Tsai et al. [13], in which the concept of CIP was introduced, all four patients with CIP were subjected to D&C.
Considering this, we believe that our study is significant in that it is the first to deliberately adopt conservative management as the treatment method after accurate diagnosis of CIP using serial transvaginal ultrasonography.
In addition, all cases yielded favorable neonatal outcomes, except for one case in which pregnancy was terminated at approximately 19 weeks at the patient’s request. All 5 mothers exhibited a good postoperative course with no high-risk obstetric complications and were successfully discharged from the hospital.
Two major complications may occur during pregnancy maintenance in mothers with CIP. The first is vaginal bleeding and preterm labor. In this study, repeated vaginal bleeding was observed in three of the five mothers. Case D was excluded because the mother elected to terminate the pregnancy at 19 weeks, whereas the other two cases experienced preterm labor that required the use of tocolytics. According to three relatively recently published case reports of CIP, repetitive vaginal bleeding required multiple hospitalizations and the use of tocolytics and steroids after hospitalization [11].
The second major complication is massive intraoperative or postpartum bleeding due to placenta previa or placenta accreta spectrum, including placenta accreta, increta, and percreta [14]. In all cases, we secured the A-line and C-line by consulting the anesthesiologist and preparing for mass transfusion, hysterectomy, and ICU care before surgery. Hysterectomy was performed in three of five cases, and intraoperative transfusion was performed immediately with pre-prepared blood; postoperative laboratory findings were considered stable. In the case of hysterectomy, the patient was admitted to the ICU for 1–2 days, managed, and discharged within 1 week with no postoperative complications. The possibility of massive bleeding and death was sufficiently explained to the patients and guardians in advance; the consent process made cooperation possible, which aimed to prevent delays in the series of required treatment processes.
In our experience, in cases of CIP in mothers who have undergone difficult pregnancies due to older age and infertility, several mothers and guardians wanted to maintain the pregnancy when treatment options were provided. Therefore, it was empirically confirmed that if CIP is accurately diagnosed, both the mother and the fetus can be healthy without unconditional termination. Of course, the outcomes are based on a thorough explanation, acknowledgment of all possibilities, and consent of all mothers and their spouses. Sufficient psychological support and counseling during the entire pregnancy and interdisciplinary cooperation among medical staff, such as those in radiology and anesthesia, are required.
Although this was a retrospective observational study and included a small number of cases at a single center, it presents a proof of concept for a new treatment paradigm and underscores the need for an independent diagnosis of CIP in LLIEP using ultrasound. We hope that successful delivery in these cases of CIP will provide an alternative option for CIP treatment to physicians treating infertility in women of advanced maternal age worldwide.
Go to : 
Notes
Ethical approval
This study was approved by a certified Institutional Review Board (DSMC 2020-12-008-005).
Funding information
The authors did not receive support from any organization for the submitted work.
Go to : 
References
1. Takeda A, Koike W, Hayashi S, Imoto S, Nakamura H. Cervico-isthmic pregnancy: early diagnostic imaging and successful dual therapy for uterine-sparing treatment. J Minim Invasive Gynecol. 2015; 22:678–83.
2. Sakai A, Fujita Y, Yumoto Y, Fukushima K, Kobayashi H, Wake N. Successful management of cervico-isthmic pregnancy delivered at term. J Obstet Gynaecol Res. 2013; 39:371–4.
3. Kayem G, Deis S, Estrade S, Haddad B. Conservative management of a near-term cervico-isthmic pregnancy, followed by a successful subsequent pregnancy: a case report. Fertil Steril. 2008; 89:1826.e13–5.
4. Avery DM, Wells MA, Harper DM. Cervico-isthmic corporeal pregnancy with delivery at term: a review of the literature with a case report. Obstet Gynecol Surv. 2009; 64:335–44.
5. Honda T, Hasegawa M, Nakahori T, Maeda A, Sai R, Takata H, et al. Perinatal management of cervicoisthmic pregnancy. J Obstet Gynaecol Res. 2005; 31:332–6.
6. Iloabachie GC, Igwegbe AO, Izuora KL. Cervico-isthmic twin pregnancy carried to 37 weeks. Int J Gynaecol Obstet. 1993; 40:59–61.
7. Itakura A, Okamura M, Ohta T, Mizutani S. Conservative treatment of a second trimester cervicoisthmic pregnancy diagnosed by magnetic resonance imaging. Obstet Gynecol. 2003; 101(5 Pt 2):1149–51.
8. Souter DJ, Roberts AB, Stables S. Cervico-isthmic pregnancy with placenta percreta ending in a livebirth. Aust N Z J Obstet Gynaecol. 1995; 35:453–6.
9. Jelsema RD, Zuidema L. First-trimester diagnosed cervico-isthmic pregnancy resulting in term delivery. Obstet Gynecol. 1992; 80(3 Pt 2):517–9.
10. Mesogitis SA, Daskalakis GJ, Doublis DG, Antsaklis AJ, Papantoniou NE, Michalas SP. Cervico-isthmic pregnancy: an extremely rare case diagnosed during labour. Eur J Obstet Gynecol Reprod Biol. 2001; 98:251–2.
11. Strobelt N, Locatelli A, Ratti M, Ghidini A. Cervico-isthmic pregnancy: a case report, critical reappraisal of the diagnostic criteria, and reassessment of the outcome. Acta Obstet Gynecol Scand. 2001; 80:586–8.
12. Oyelese Y, Elliott TB, Asomani N, Hamm R, Napoli L, Lewis KM. Sonography and magnetic resonance imaging in the diagnosis of cervico-isthmic pregnancy. J Ultrasound Med. 2003; 22:981–3.
13. Tsai S, Huang KH, Ou YC, Hsu TY, Wang CB, Chang MS, et al. Low-lying-implantation ectopic pregnancy: a cluster of cesarean scar, cervico-isthmus, and cervical ectopic pregnancies in the first trimester. Taiwan J Obstet Gynaecol. 2013; 52:505–11.
14. Pegu B, Thiagaraju C, Nayak D, Subbaiah M. Placenta accreta spectrum-a catastrophic situation in obstetrics. Obstet Gynecol Sci. 2021; 64:239–47.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download