Abstract
Objective
To describe the roadmapping technique and our three-year experience in the management of intracranial aneurysms in the hybrid operating room.
Methods
We analyzed all patients who underwent surgical clipping for cerebral aneurysms with the roadmapping technique from January 2017 to September 2019. We report demographic, clinical, and morphological variables, as well as clinical and radiological outcomes. We further describe three illustrative cases of the technique.
Results
A total of 13 patients were included, 9 of which (69.2%) presented with subarachnoid hemorrhage, with a total of 23 treated aneurysms. All patients were female, with a mean age of 47.7 years (range 31-63). All cases were anterior circulation aneurysms, the most frequent location being the ophthalmic segment of the internal carotid artery (ICA) in 11 cases (48%), followed by posterior communicating in 8 (36%), and ICA bifurcation in 2 (8%). Intraoperative clip repositioning was required in 9 aneurysms (36%) as a result of the roadmapping technique in the hybrid operating room. There were no residual aneurysms in our series, nor reported mortality.
Conclusions
The roadmapping technique in the hybrid operating room offers a complementary tool for the adequate occlusion of complex intracranial aneurysms, as it provides a real time fluoroscopic-guided clipping technique, and clip repositioning is possible in a single surgical stage, whenever a residual portion of the aneurysm is identified. This technique also provides some advantages, such as immediate vasospasm identification and treatment with intra-arterial vasodilators, balloon proximal control for certain paraclinoid aneurysms, and simultaneous endovascular treatment in selected cases during a single stage.
Intraoperative imaging has significantly evolved over the last three decades in neurovascular surgery. Nowadays, there is a wide range of intraoperative resources, ranging from intraoperative digital substraction angiography (iDSA), micro-Doppler, neuronavigation systems, magnetic resonance imaging and fluorescent dyes. These methods are used to evaluate vascular anatomy and confirm adequate treatment during surgery, particularly in complex vascular pathologies in order to perform safer and more efficient procedures [8].
The recently described “hybrid operating room” (HOR) concept integrates microsurgical procedures with the use of iDSA for simultaneous endovascular diagnosis and treatment in the same operation room (OR), optimizing treatment and avoiding the need to transfer the patient from the OR to the angio-suite, among other benefits [4,5,17]. The digital roadmapping technique, available in the HOR, is an intraoperative adjunct that uses a video mixer to superimpose an opacified digital subtraction angiographic image of the vessel onto live fluoroscopic images, providing real-time guidance of the catheter and other surgical instruments in motion within the fluoroscopic view [14,24,25].
In 2007, Ayad et al. first described this technique as an alternative intraoperative navigation tool for the microsurgical resection of arteriovenous malformations (AVMs) and dural fistulas [2]. However, its use in the surgical treatment of intracranial aneurysms has not been widely described. In the present study, we report our experience and describe the technical nuances of roadmapping-assisted surgery during iDSA in the HOR for the treatment of intracranial aneurysms.
We performed a retrospective review of all patients with intracranial aneurysms that were surgically treated in the HOR at our center using the roadmapping technique from January 2017 to September 2019. Hunt & Hess (HH) and modified Fisher (mFisher) grades [7,13] were documented in case of subarachnoid hemorrhage (SAH). Preoperative imaging studies included a computed tomography (CT) scan, CT angiography (CTA) and digital subtraction angiography (DSA), and aneurysm size was categorized according to dome-to-neck distance Yaşargil classification [27]. Surgical intervention was performed in the HOR when deemed appropriate by the treating neurosurgeon in patients with complex aneurysm anatomy, multiple aneurysms, or certain paraclinoid aneurysms requiring extradural proximal control. All surgical and endovascular procedures were authorized via written informed consent and were performed by a skull base and neurovascular surgeon (senior author, JLGA) and by a neurointerventional radiologist (co-author, MAZC). Patients were followed-up as outpatients every 3-6 months by both teams. The functional outcome was determined 3 months after surgery using the modified Rankin Scale (mRS) [20]. We considered a mRS ≥2 as an unfavorable outcome.
Demographic and clinical variables of the population, as well as morphologic features of the treated aneurysms are reported. Continuous variables are summarized as means or medians according to their distribution determined by the Shapiro-Wilk test, and categorical variables are reported as percentages.
The HOR is an area capable of offering a setting for microsurgical dissection and clipping combined with simultaneous intraoperative endovascular diagnosis and treatment. The HOR can be assembled in the angio suite, equipped with a multi-axial uniplanar angiograph (Artis zeego multiaxis system, Siemens, Germany), or in the conventional OR using a monoplanar fluoroscope (Arcadis, Siemens, Germany) able to perform a DSA and roadmapping images. The latter is limited to perform three-dimensional reconstructions and endovascular treatment such as coiling or stenting, unlike the angio suite HOR.
The patient was placed supine on a radiolucent operating table with the head positioned in a headrest. The team wore suitable radiation protective equipment throughout the procedure. Once the femoral access was prepped and draped, a 6 Fr femoral introducer sheath (Terumo, Japan) was placed in a femoral artery using the Seldinger technique. The femoral introducer sheath was continuously perfused with a 500 ml/hr heparinized saline solution (1000 USP units of heparin sodium were added to 1000 ml of normal saline). The endovascular team used a 6 Fr 40º curved chaperon catheter (Terumo, Japan) with a 0.035”/0.038” hydrophilic guidewire (Terumo, Japan) to navigate through the arteries up to the aortic arch. Femoral artery access was preferred over radial access as it allows for more versatility and working space for both teams in the OR. Initial DSA images were obtained using hand injections of iomerol contrast (Iomeron 300/100 ml, Bracco, Italy). Once multiple orthogonal and three-dimensional images identifying the anatomical structure of the aneurysm (Syngo, DynaCT, Siemens Healthcare Solutions, Erlangen, Germany) were acquired, the neurosurgical team prepped and draped the skin and performed the cranial approach. The C-arm and the microscope (Pentero 900, Carl Zeiss, Germany) are then placed as depicted in Fig. 1. The position of the C-arm may vary depending on the best projection image of the aneurysm and this position can be changed during surgery to allow for an ergonomic position of the surgeon, who is being surrounded by the C-arm. A pterional approach with internal orbitotomy was performed in most cases, followed by an intradural or extradural anterior clinoidectomy in cases of paraclinoid and posterior communicating aneurysms. After dural opening and initial dissection, the endovascular team obtained an angiographic image that will be then used as roadmap. Then, the roadmap images were intermittently displayed after the neurosurgeon dissected the neck of the aneurysm. This way, the neurosurgeon, aided by realtime fluoroscopic guidance with roadmapping confirms the boundaries of the aneurysm neck and the projection of the aneurysm dome based on the position of a dissector or the bipolar forceps and then dissects around the aneurysm under microscopic view to obtain further anatomical information. This way, fluoroscopy is not employed during microdissection. In some cases where proximal control was deemed complex, as it occurs in some paraclinoid aneurysms, an intra-arterial balloon (Scepter, MicroVention, Terumo, Tokyo, Japan; Hyperglide, Medtronic, Minneapolis, MN, USA) was inflated for this purpose when requested by the neurosurgeon. Dissection and clipping of the aneurysm were performed under direct vision on the surgical microscope, and the confirmed by roadmapping, providing a complete picture to approximate a proper clip position prior to definitive occlusion. The endovascular team performed an iDSA after clipping to confirm the complete exclusion of the aneurysm. If a residual aneurysm was identified, the clip was repositioned assisted by roadmapping until the aneurysm was completely excluded. After final repositioning, iDSA was performed in different orthogonal projections and 3D reconstructions to document the success of the aneurysm occlusion and to treat vasospasm – if identified – with intra-arterial vasodilators.
Thirteen patients were included in the study, all of them were females. Table 1 depicts demographic and clinical characteristics of our population. Mean age was 47.7, ranging from 31 to 63 years. Cardiovascular comorbidities included the following: hypertension in 5 patients (38%), overweight or obesity in 4 patients (31%). Other risk factors are described in Table 1. The most common symptom was headache in 11 patients (84.5%), followed by altered consciousness in 3 (23%), visual impairment in 2 (15.3%), seizures in 1 (7.6%) and cranial nerve palsy in 1 (7.6%). All patients had a preoperative mRS ≤2 points.
Nine patients (69.2%) presented with SAH, and four (30.8%) had unruptured aneurysms. One patient presenting with HH grade 4, was diagnosed with acute hydrocephalus and was accordingly treated with a ventricular drain. Vasospasm was documented in six patients either by iDSA or by transcranial Doppler after the procedure.
A total of twenty-three aneurysms were diagnosed angiographically by the endovascular team, from which 12 (52%) were paraclinoid, 8 (36%) posterior communicating, 1 (4%) in middle cerebral artery (MCA) bifurcation, 1 (4%) in internal carotid artery (ICA) bifurcation, and 1 (4%) medial-projecting superior hypophyseal. Eight patients (61.5%) harbored multiple aneurysms. Regarding size, 1 (4%) was a baby aneurysm (<2 mm), 17 (73%) were small (2–5 mm), 3 (14%) were large 16–25 mm, and 2 (9%) were giant (>25 mm). Of note, 2 out of 23 aneurysms (8%) were residual aneurysms from previously clipped cases in the conventional OR. Aneurysm characteristics are depicted in Table 2.
Seventeen aneurysms (73%) were clipped through a pterional approach. From these, four (17%) were treated in a conventional HOR and 13 (56%) in the angio-suite HOR. Coil embolization was performed during the same procedure in 4 distant aneurysms (17%) after the surgical stage. One case (4%), the superior hypophyseal aneurysm, was clipped through an endoscopic endonasal approach. Only one ICA bifurcation bleb aneurysm was not clipped and wrapping technique was used instead.
Nine (69%) cases required clip repositioning due to a residual aneurysm (Sindou 1 and 2) or parental artery subtotal occlusion detected by the iDSA, in which cases, repositioning was immediately performed during the same surgical stage, deeming unnecessary a second surgery. In all cases, complete occlusion and parental vessel patency were confirmed at the final angiographic series.
Postoperative morbidity was present in 3 patients (23%), attributed to delayed cerebral ischemia due to cerebral vasospasm. Two patients (15.3%) underwent a decompressive hemicraniectomy due to refractory intracranial hypertension, days after the treatment of the aneurysm, as a result of vasospasm-induced cerebral infarction, resulting in an unfavorable functional outcome (mRS ≥2 at three-months follow-up). Surgical outcomes are summarized in Table 3. All cases are summarized in Table 4. There was no mortality in our series up until last follow-up.
A 40-year-old female presented with a 6-months history of left monocular blindness and a recent onset of acute severe headache associated with transient loss of consciousness (HH grade 2). CT scan showed an mFisher grade 4 SAH. 3D CTA revealed a left-sided giant ophthalmic aneurysm (25 mm) and a right-sided posterior communicating small aneurysm (3 mm). On preoperative DSA, the ophthalmic aneurysm revealed a wide neck with a triangular-shaped dorsally projecting dome, and the posterior communicating aneurysm had a posteromedial projection (Fig. 2). A left pterional approach with extradural anterior clinoidectomy was performed followed by clipping and remodeling of both aneurysms (Fig. 3), a residual aneurysm in the ophthalmic aneurysm was found in the iDSA (Fig. 4A), requiring clip repositioning, which was more easily performed after aneurysm remodeling. iDSA after clips repositioning revealed complete occlusion of both aneurysms (Fig. 4B, C). The patient was discharged without further complications. On follow-up, a favorable functional outcome (mRS 1) was documented but visual function remained unchanged.
A 51-year-old female presented with a sudden onset severe headache (HH grade 2). The CT scan showed localized SAH in the right carotid cistern (mFisher grade 2). On DSA, two right-sided Barami IB and II small aneurysms were found (Fig. 5A, B). We performed a right pterional approach with extradural anterior clinoidectomy. Proximal balloon control on the cavernous segment of the ICA was performed and clipping of both aneurysms was carried out (Fig. 5C, D). Clip repositioning was needed due to residual aneurysm during iDSA (Fig. 5E), and complete occlusion was achieved with no postoperative complications (Fig. 5F). On follow-up, the patient remained asymptomatic with a mRS of 0.
Even though the roadmapping technique has been available for four decades, Ayad et al. [2] first reported 38 cases where it was used during open surgery mainly for arteriovenous malformation, dural fistulae, and one distal MCA mycotic aneurysm. Although they described it as an alternative intraoperative tool for neurovascular surgery to verify the location of the lesion preoperatively and immediately after dural opening, they did not describe its use during the microdissection or the resection of the lesion. The present paper describes the roadmapping technique in the microsurgical treatment of intracranial aneurysms. This technique allows the neurosurgeon to navigate through the aneurysm anatomy and surrounding vessels with real-time assistance from angiographic images during the microsurgical dissection and treatment.
In our series, roadmapping technique was a valuable complementary resource during microdissection of complex ICA aneurysms (mainly for ventral/medial paraclinoid aneurysm) where the neck could not be clearly identified. This technique offered accurate location of proximal and distal neck boundaries, real-time image guidance and continuous feedback on the orientation of the clip blades during clip placement. Similarly, iDSA allowed a prompt identification of any residual aneurysm or parental vessel occlusion, calling for immediate clip repositioning. Overall, these features are considered the mainstay of this technique, thus culminating in no residual aneurysms or parental vessel occlusions in our series.
Aneurysm location is of paramount importance when planning the surgery, as it can determine the complexity of the approach and proximal control. In this series, the only case of an MCA aneurysm was a giant aneurysm that caused distorted local anatomy. All other treated aneurysms were located in the ICA, and shared common characteristics: giant, multiple, medial/ventral paraclinoid, and bilateral aneurysms. However, location alone should not determine the usefulness of this adjunct.
Regarding surgical morbidity, those patients who presented a poor functional outcome during follow-up had SAH with severe vasospasm resulting in delayed cerebral ischemia and malignant infarctions requiring decompressive hemicraniectomy (15.3%), followed by tracheostomy and gastrostomy. Interestingly, our rates of vasospasm were lower than those previously reported [2], but the need for decompressive hemicraniectomy was higher [10]. Both patients had mRS of ≥2 at three-months follow-up despite aggressive treatment against vasospasm and intracranial hypertension.
Several tools and techniques are available to increase safety and efficacy of the microsurgical treatment of intracranial aneurysms, such as indocyanine green (ICG) video angiography, intraoperative micro-Doppler, intraoperative CT scan, intraoperative magnetic resonance imaging (MRI), and the use of an HOR for iDSA for diagnostic and therapeutic purposes [5,8,16,18,19,26]. After the introduction of ICG video angiography, the use of iDSA has decreased, since the former allows the neurosurgeon to confirm the occlusion of the aneurysm and the patency of the parental vessels without the requirement of DSA equipment or the need of an additional invasive procedure. Nowadays, ICG video angiography is used commonly for intracranial/spinal AVMs and arteriovenous fistulas, but its use is limited by a narrow microscope field [6,9,15,21]. Instead, iDSA and roadmapping have the advantage of predefined real-time images to assess vascular architecture during the dissection and treatment of the aneurysm beyond the microscope’s field of view (i.e., medial/ventral paraclinoid aneurysm, carotid cavum aneurysms and contralateral aneurysm).
Other resources commonly used for tumor resection, such as intraoperative MRI and CT can also be used in vascular surgery to delineate the extent of resection of AVMs or cavernous malformations. However, its use is limited in the treatment of intracranial aneurysms [1,3,8,22]. Similarly, intraoperative micro-Doppler is a noninvasive and reliable tool for the evaluation of the parental and adjacent vessels in an aneurysmal clipping, but it is an operator-dependent study, and it cannot show any residual aneurysm [23]. Nowadays, the HOR concept has been widely adopted in neurosurgical centers around the world. We consider that the HOR provides a variety of assets available during the surgical procedure, allowing us to perform not only intraoperative DSA but to perform hybrid treatment of intracranial aneurysms [5,18]. Recently, multiple reports have addressed the advantages of an HOR in different cerebrovascular diseases with acceptable outcomes and have included most of the benefits we mentioned before [4,11,18,19]. Gruber et al. revised all available intraoperative techniques, concluding that these methods are complementary rather than competitive in aneurysm surgery, a view with which the authors certainly agree [9].
Some disadvantages of iDSA and the roadmapping technique include the comfort of the surgical team, the need for additional equipment including a radiolucent operating table, the required infrastructure of an HOR (or an OR equipped with a portable fluoroscope capable of performing roadmapping), and the availability of an experienced staff. Complications related to the procedure imply those previously reported for any angiographic procedure, ranging from 0.5 to 3.29% [11,12]. Furthermore, radiation exposure may be a concern; however, safety for the medical staff must be assured by maintaining the duration of the image acquisition and roadmapping to the minimum necessary and making certain of the continuous use of protective equipment. Importantly, the angiographic series obtained for roadmapping are acquired with the surgical team away from the C-arm. Then, when roadmapping is displayed, the fluoroscope is briefly activated at the request of the surgeon and only after dissection and clip positioning were initially performed under direct microscopic view, thus reducing radiation exposure.
With the aid of roadmapping, an adequate assessment of vascular anatomy can be obtained after careful dissection has been made in order to define anatomical landmarks and estimate initial clip positioning. One consideration worth mentioning is that roadmapping images are not an exact reflection of vascular anatomy since they are based on angiographic series acquired immediately before they are displayed and lack real-time feedback. Thus, any tissue shift during dissection and clip positioning should be taken into account in order not to misinterpret the actual anatomy and potentially lead to suboptimal results or catastrophic complications. Therefore, rather than an alternative to standard techniques, its use can be viewed as a complementary navigation system using intraoperative updated images.
The use of iDSA with roadmapping technique in an HOR for complex aneurysms (e.g., ventral/medial paraclinoid aneurysms, multiple aneurysms with a contralateral lesion for a planned unilateral approach and certain residual aneurysms) offers not only diagnostic DSA as needed, but also real-time image guidance during microdissection and clip positioning or repositioning, the latter being the main advantage for this technique. The use of roadmapping over conventional iDSA requires no additional equipment and provides a complementary tool that should be used in addition to iDSA to provide a safer clipping technique. Furthermore, the proximal control offered by an intra-arterial balloon, as well as the possibility of concomitant aneurysm embolization in selected cases, makes this intraoperative adjunct a valuable tool for vascular surgery. Another advantage of this modality is the ability to diagnose and treat vasospasm immediately after aneurysm occlusion has been confirmed. Besides from achieving these goals in a single stage, the patient’s transfer among different facilities is avoided, thus preventing further associated complications.
We acknowledge that this modality may not be universally available and should thus be tailored to specific cases. In our series, the included patients fullfilled certain characteristics that were thought to anticipate a complex procedure, such as size, previous treatment, dome projection, among others. Each case was thoroughly discussed among the endovascular and neurosurgical teams, and a consensual decision was chosen in every one of them. We acknowledge that some might opt for a pure endovascular treatment in particular cases, however due to financial and bureaucratic limitations, this is not always feasible at our institution, and if not done microsurgically, the treatment could be delayed, thus putting our patients’ lives under unnecessary risks. It would be interesting to describe this technique in different groups of aneurysms, such as posterior circulation aneurysms, which are generally not treated surgically at our center. Due to the high heterogeneity of intracranial aneurysms, it would be unreasonable to determine strict selection criteria for this surgical adjunct; therefore, the ideal strategy should be agreed upon by an experienced multidisciplinary team on an individualized basis.
The iDSA and roadmapping technique is a valuable intraoperative adjunct that promotes safe and efficient aneurysm microdissection and clipping by delineating the boundaries of the aneurysm and the parental vessels in real-time, particularly in technically demanding aneurysms such as, medial/ventral paraclinoid aneurysms or those requiring extradural proximal control, carotid cavum aneurysms, giant, and contralateral aneurysms. Excellent clinical and surgical outcomes can be obtained with the proposed technique, with no procedure-related morbidity and no residual aneurysms. The main advantages of this technique are that it provides a real time fluoroscopic-guided clipping technique, and clip repositioning is possible in a single surgical stage, whenever a residual portion of the aneurysm is identified.
REFERENCES
1. Albayrak B, Samdani AF, Black PM. Intra-operative magnetic resonance imaging in neurosurgery. Acta Neurochir (Wien). 2004; Jun. 146(6):543–56. discussion 557.
2. Ayad M, Ulm AJ, Yao T, Eskioglu E, Mericle RA. Real-time image guidance for open vascular neurosurgery using digital angiographic roadmapping. Neurosurgery. 2007; Sep. 61(3 Suppl):55–61. discussion 61-2.
3. Bisdas S, Roder C, Ernemann U, Tatagiba MS. Intraoperative MR imaging in neurosurgery. Clin Neuroradiol. 2015; Oct. 25 Suppl 2:237–44.
4. Choi E, Lee JY, Jeon HJ, Cho BM, Yoon DY. A hybrid operating room for combined surgical and endovascular procedures for cerebrovascular diseases: a clinical experience at a single centre. Br J Neurosurg. 2019; Oct. 33(5):490–4.
5. Dammann P, Jägersberg M, Kulcsar Z, Radovanovic I, Schaller K, Bijlenga P. Clipping of ruptured intracranial aneurysms in a hybrid room environment—a case-control study. Acta Neurochir (Wien). 2017; Jul. 159(7):1291–8.
6. Friedman JA, Kumar R. Intraoperative angiography should be standard in cerebral aneurysm surgery. BMC Surg. 2009; Apr. 9:7.
7. Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery. 2006; Jul. 59(1):21–7.
8. Goren O, Monteith SJ, Hadani M, Bakon M, Harnof S. Modern intraoperative imaging modalities for the vascular neurosurgeon treating intracerebral hemorrhage. Neurosurg Focus. 2013; May. 34(5):e2.
9. Gruber A, Dorfer C, Standhardt H, Bavinzski G, Knosp E. Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery. 2011; Mar. 68(3):657–73.
10. Güresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus. 2009; 26(6):e4(1–8).
11. Heiserman JE, Dean BL, Hodak JA, Flom RA, Bird CR, Drayer BP, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994; Sep. 15(8):1401–7. discussion 1408-11.
12. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981; Feb. 138(2):273–81.
13. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; Jan. 28(1):14–20.
14. Jones DW, Stangenberg L, Swerdlow NJ, Alef M, Lo R, Shuja F, et al. Image fusion and 3-dimensional roadmapping in endovascular surgery. Ann Vasc Surg. 2018; Oct. 52:302–11.
15. Killory BD, Nakaji P, Gonzales LF, Ponce FA, Wait SD, Spetzler RF. Prospective evaluation of surgical microscope–integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009; Sep. 65(3):456–62; discussion 462.
16. Kotowski M, Sarrafzadeh A, Schatlo B, Boex C, Narata AP, Pereira VM, et al. Intraoperative angiography reloaded: a new hybrid operating theater for combined endovascular and surgical treatment of cerebral arteriovenous malformations: a pilot study on 25 patients. Acta Neurochir (Wien). 2013; Nov. 155(11):2071–8.
17. Liao CH, Chen WH, Lee CH, Shen SC, Tsuei YS. Treating cerebrovascular diseases in hybrid operating room equipped with a robotic angiographic fluoroscopy system: level of necessity and 5-year experiences. Acta Neurochir (Wien). 2019; Mar. 161(3):611–9.
18. Murayama Y, Arakawa H, Ishibashi T, Kawamura D, Ebara M, Irie K, et al. Combined surgical and endovascular treatment of complex cerebrovascular diseases in the hybrid operating room. J Neurointerv Surg. 2013; Sep. 5(5):489–93.
19. Murayama Y, Irie K, Saguchi T, Ishibashi T, Ebara M, Nagashima H, et al. Robotic digital subtraction angiography systems within the hybrid operating room. Neurosurgery. 2011; May. 68(5):1427–32. discussion 1433.
20. Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009; Oct. 40(10):3393–5.
21. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003; Jan. 52(1):132–9.
22. Schwartz RB, Hsu L, Wong TZ, Kacher DF, Zamani AA, Black PM, et al. Intraoperative MR imaging guidance for intracranial neurosurgery: experience with the first 200 cases. Radiology. 1999; May. 211(2):477–88.
23. Siasios I, Kapsalaki EZ, Fountas KN. The role of intraoperative micro-Doppler ultrasound in verifying proper clip placement in intracranial aneurysm surgery. Neuroradiology. 2012; 54(10):1109–18.
24. Söderman M, Babic D, Homan R, Andersson T. 3D roadmap in neuroangiography: technique and clinical interest. Neuroradiology. 2005; Oct. 47(10):735–40.
25. Tobis J, Johnston WD, Montelli S, Henderson E, Grad WRE, Bauer B, et al. Digital coronary roadmapping as an aid for performing coronary angioplasty. Am J Cardiol. 1985; Aug. 56(4):237–41.
26. Xin C, Zhang J, Li Z, Xiong Z, Yang B, Wu X, et al. Treatment of giant cavernous aneurysm in an elderly patient via extracranial–intracranial saphenous vein bypass graft in a hybrid operating room: a case report. Medicine (Baltimore). 2018; Apr. 97(14):e0295.
27. Yasargil MG. Microneurosurgery: I. Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. New York, NY: Thieme;1984. p. 280.
Fig. 1.
Organization for the hybrid operation room involving endovascular team (ET), neurosurgery, anesthesiology and nursing teams.
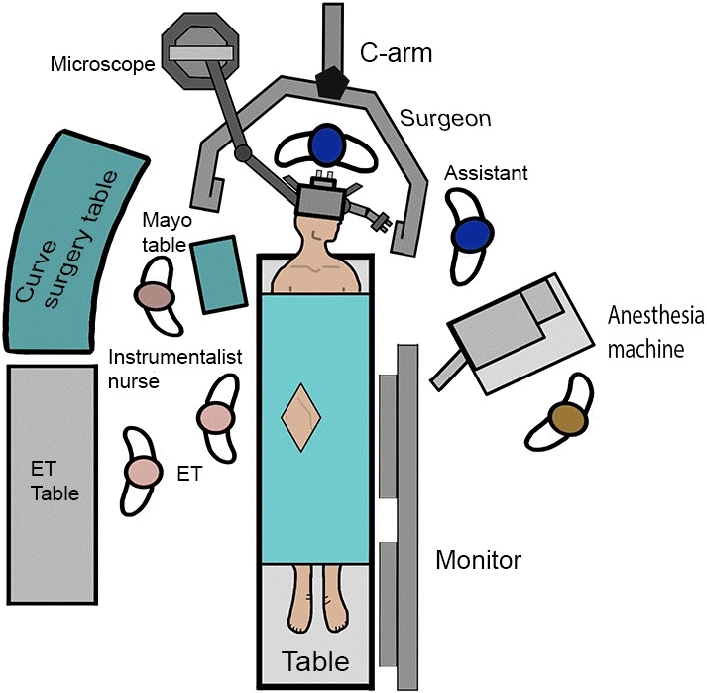
Fig. 2.
Case #8. Digital substraction angiography (DSA) preoperative images. (A) anteroposterior (AP) projection of the left carotid axis shows a giant (25 mm) ophthalmic aneurysm (green arrowhead), (B) lateral projection of the right carotid axis shows a small (3 mm) posterior communicating aneurysm (yellow arrowhead).
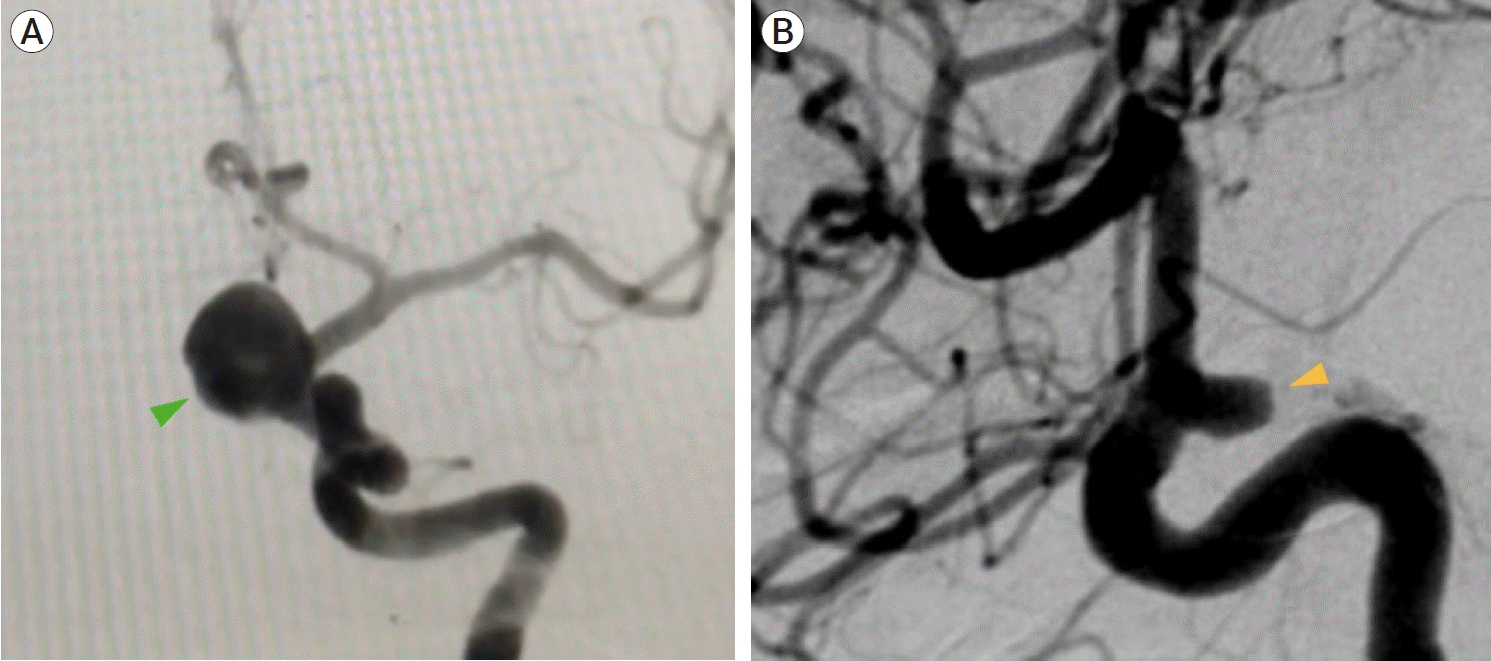
Fig. 3.
Case #8. Intraoperative images. Left pterional approach: (A) Aneurysm dissection in which the aneurysm dome (green arrowhead) is dorsally projected. After opening the opticocarotid cistern, the optic nerve (ON) and internal carotid artery (ICA) can be seen. (B) After the aneurysm neck and dome are exposed, the clip blades (*) are placed around the aneurysm´s neck. (C) Intraoperative roadmapping technique in which initial clip position is guided by real-time imaging.
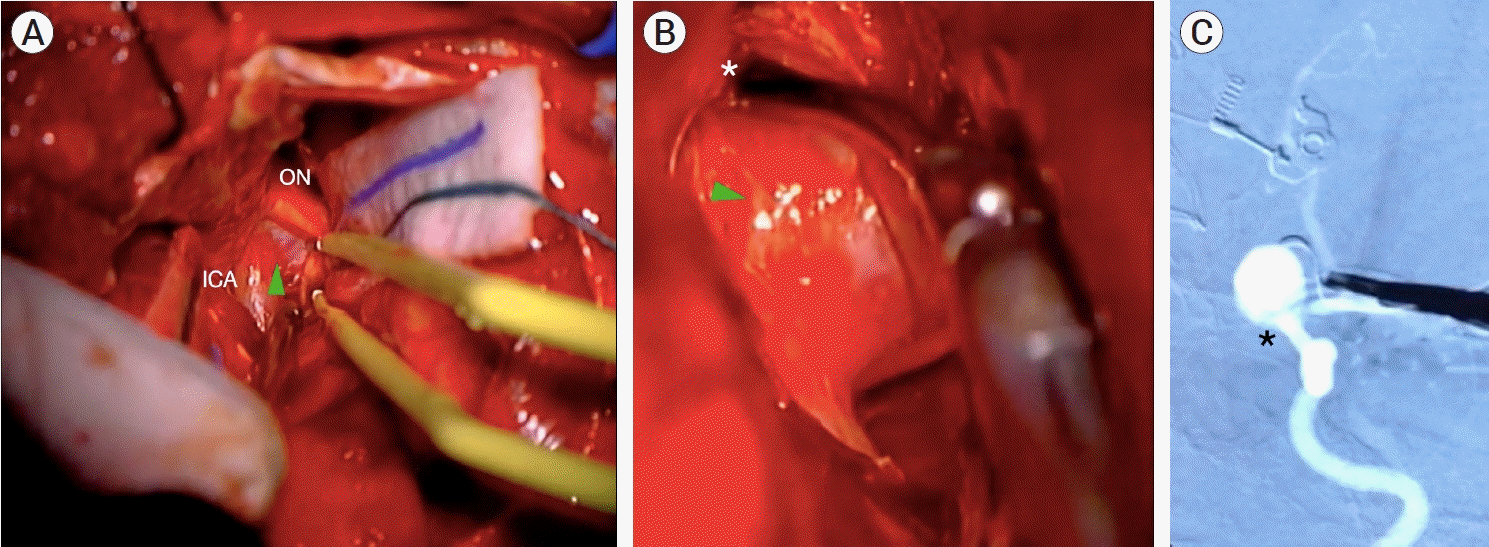
Fig. 4.
Case #8. iDSA images. (A) Left ICA, AP projection, a residual ophthalmic aneurysm (black arrowhead) is observed. (B) Left ICA, AP projection, after clip repositioning: complete aneurysm occlusion with no remnant is observed. (C) Right ICA, lateral projection: complete occlusion of the posterior communicating aneurysm. ICA, internal carotid artery; AP, anteroposterior
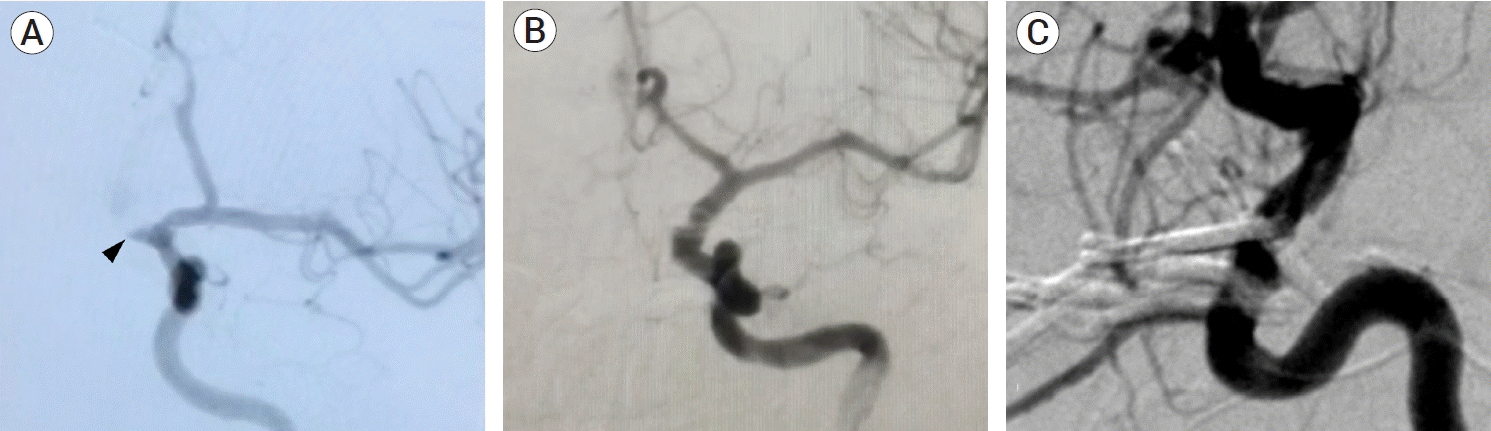
Fig. 5.
Case #13. Preoperative DSA images. (A) Lateral projection of the right ICA and (B) 3D DSA reconstruction in which two right-sided ophthalmic aneurysms are observed, a Barami type Ib (green arrowhead) and a type II (yellow arrowhead). Intraoperative images. (C) After opening of the opticocarotid cistern, a 90º angled fenestrated clip was allocated around the type II aneurysm´s neck, the dome has a ventral projection, and behind the ICA, out of the microscope field. (D) Roadmapping image during clip positioning. The clip blades (*) completely surround the aneurysm´s neck, while a proximal control balloon (black arrow) on the cavernous segment of the ICA is placed. Lateral iDSA projection. (E) A residual aneurysm is observed (black arrowhead). (F) Complete occlusion in control iDSA after clip repositioning is achieved. Note the relationship of the aneurysms with the ophthalmic artery (OA). DSA, digital subtraction angiography; ICA, internal carotid artery; ON, optic nerve

Table 1.
Demographic and clinical characteristics
Table 2.
Aneurysm characteristics
| Variable | N | Percentage (%) | |
|---|---|---|---|
| SAH | Yes | 9 | 69.2 |
| No | 4 | 30.8 | |
| Circulation | Anterior | 13 | 100 |
| Posterior | 0 | 0 | |
| Hunt & Hess grade | 1 | 2 | 22.2 |
| 2 | 6 | 66.6 | |
| 3 | 0 | 0 | |
| 4 | 1 | 11.1 | |
| Vasospasm | 6 (66.6%) | ||
| Patients with multiple aneurysms | 8 (61.5%) | ||
| Contralateral aneurysms clipped | 1 (12.5%)* | ||
| Total aneurysms diagnosed | 23 (100%) | ||
| Treated aneurysms | 22 (94%)** | ||
| Aneurysm location | Paraclinoid | 11 | 48 |
| PComm artery | 8 | 36 | |
| ICA Bifurcation | 2 | 8 | |
| Superior hypophyseal | 1 | 4 | |
| MCA Bifurcation | 1 | 4 | |
| Side | Right | 14 | 61 |
| Left | 9 | 39 | |
| Size | <2 mm (baby) | 1 | 4 |
| 2-15 mm (small) | 17 | 73 | |
| 16-25 (big) | 3 | 14 | |
| >25 mm (giant) | 2 | 9 | |
| Treatment modality | - Transcranial clipping conventional HOR | 4 | 18 |
| - Transcranial clipping angio-suite HOR | 13 | 58 | |
| - Transcranial clipping + embolization with coils in angio-suite HOR | 4 | 18 | |
| - Endonasal clipping in angio-suite HOR | 1 | 5 | |
| - Wrapping in angio-suite HOR | 1** | 5 | |
| Clip repositioning in HOR | 9 (69%) | ||
| Residual aneurysms treated in HOR | 2 (8%) | ||
Table 3.
Surgical outcomes
Table 4.
Summary of procedures performed in hybrid room




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download