Abstract
Moyamoya disease (MMD) is a rare progressive steno-occlusive cerebrovascular disorder. Currently, revascularization surgery is used as optimal treatment to overcome MMD. However, revascularization for MMD has reported several complications. Also, iatrogenic complications such as pseudoaneurysms formation or dural arteriovenous fistulas (dAVFs) formation—has been identified in rare cases after the surgical intervention for revascularizations.
We describe two cases. In first case, the patency of the anastomosis site was good and saccular type pseudoaneurysm formation was found at parietal branch of posterior middle meningeal artery (MMA) in transfemoral cerebral angiography (TFCA) performed on the twelfth day after surgery. We decided to treat pseudoaneurysm by endovascular embolization the next day, but the patient was shown unconsciousness and anisocoria during sleep at that day. Computed tomography showed massive subdural hemorrhage at the ipsilateral side, thus we performed decompressive craniectomy and hematoma evacuation.
In second case, the patency of the anastomosis site was good and dAVF formation at right MMA was found in TFCA performed on the sixth day after surgery. We performed endovascular obliteration of the arteriovenous fistula under local anesthesia.
Pseudoaneurysm formation or dAVF formation after revascularization surgery is an exceptional case. If patients have such complications, practioner should carefully screen the patients by implementing digital subtraction angiogram to identify anatomic features; as well as consider immediate treatment in any way, including embolization or other surgery
Moyamoya disease (MMD) is a rare progressive stenoocclusive cerebrovascular disorder. Currently, revascularization surgery is used as optimal treatment to overcome MMD. However, revascularization for MMD has reported several complications including ischemic stroke, hemorrhagic stroke, hyperperfusion syndrome, and epidural hematoma. However, iatrogenic complications such as pseudoaneurysms formation or dural arteriovenous fistulas (dAVFs) formation—has been identified in rare cases after the surgical intervention for revascularizations.
We reported the following two cases: patients with hemorrhage from pseudoaneurysm formation or dural arteriovenous fistula formation who have been operated on the extracranial-intracranial arterial bypass (EIAB) surgery.
A 46-years-old female patient presented with headache and dizziness. Although she was conscious and well oriented, computed tomography (CT) angiogram found the intraventricular hemorrhage and MMD. To identify the perfusion defect, the brain Diamox single-photon emission computed tomography (SPECT) was performed. After 2 months, left EIAB surgery was first performed on the patient. The frontal branch of superficial temporal artery (STA) was used for end-to-side anastomosis with M4 of middle cerebral artery (MCA), while parietal branch of STA was used for encephalo-duroarterio-synangiosis (EDAS) and dura flaps were repositioned on brain surface. There were no unusual events such as traumatic events during surgery. In the immediate postoperative period, she was stable and had no events. The patency of the anastomosis site was good and saccular type pseudoaneurysm formation was found at parietal branch of posterior middle meningeal artery (MMA) in transfemoral cerebral angiography (TFCA) performed on the twelfth day after surgery (Fig. 1). We decided to treat pseudoaneurysm by endovascular embolization the next day, but the patient was shown unconsciousness and anisocoria during sleep at that day. CT showed massive subdural hemorrhage at the ipsilateral side, thus we performed decompressive craniectomy and hematoma evacuation. At the last follow up CT after two weeks, left MCA territory infarction could be confirmed. After one month, she was discharged from the hospital, and the patient was not followed up after discharge. At the time of discharge, mental status was alert, but global aphasia was shown, and the patient was able to gait herself about 200 meters and showed 4 points on the modified Rankin Scale.
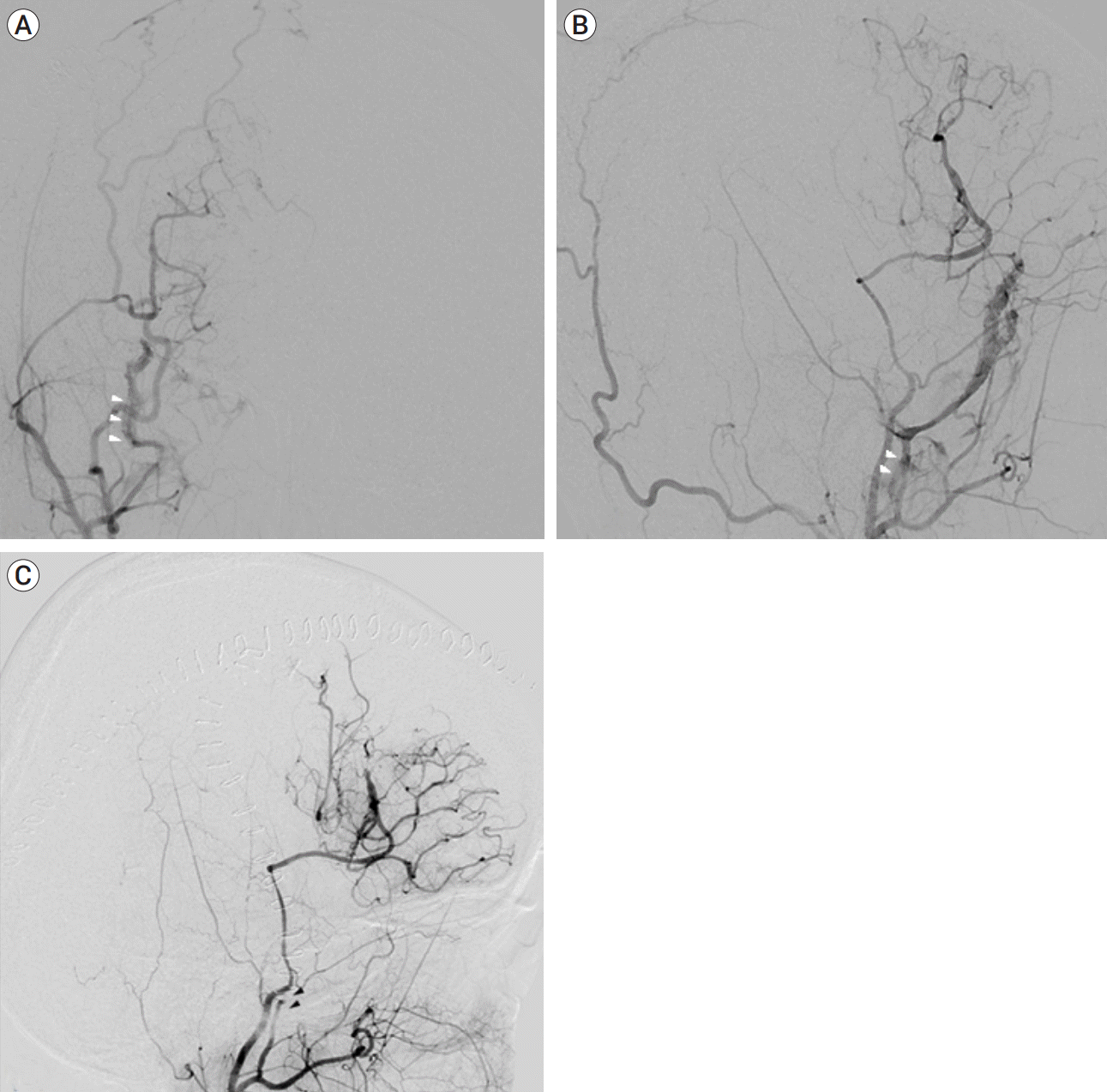
A 33-year-old female had episodic transient hemiparesis into the both sides alternately when she ran or gave strength. The brain magnetic resonance imaging and brain Diamox SPECT was performed and she was diagnosed MMD. After 2 months, the patient underwent planned right side cranial revascularization with perfusion defect by direct anastomosis by using STA– M4 of MCA and EDAS and dura flaps were repositioned on brain surface. There were no unusual events such as traumatic events during surgery. After the surgery, the patient’s symptoms were improved. The patency of the anastomosis site was good and dAVF formation at right MMA was found in TFCA performed on the sixth day after surgery (Fig. 2). Thus, we performed endovascular obliteration of the arteriovenous fistula under local anesthesia. The right external carotid artery was catheterized with a 5 French guiding catheter (Envoy, Codman Neurovascular, USA) and a microcatheter (Marathon, Medtronics, USA) was navigated into the right. In a micro-angiogram, it revealed a direct fistula of a single hole and made occlusion by injection 33% glue and lipiodol mixture of those areas. The dAVF was occluded successfully and the patient was tolerable.
There are no questions that surgical revascularization may be the most effective treatment for symptomatic ischemic MMD with perfusion defect [4,11,14]. And surgical revascularizations has direct bypass that enforces end-to-side anastomosis for STA and MCA and indirect bypass including encephaloduroarteriomyosynangiosis, encephaloduroarteriosynangiosis, encephalomyoarterio-synangiosis, and encephalomyosynangiosis [1,16].
Revascularization surgery for MMD has a higher incidence and frequency of postoperative complications than other brain surgery. In particular, hyperperfusion syndrome (21.5~50.0%) and ischemic stroke (3.2~7.7%) were well-known complications related to direct bypass. And the other complications included hemorrhagic stroke (0.7~8.0%) and epidural hematomas (4.8%) [8,12,14]. And iatrogenic complications such as pseudoaneurysm and dAVF might be occurred after revascularization surgery rarely.
Pseudoaneurysms is a rare complications of craniotomy. Pseudoaneurysms were able to develop when injury of the vessel wall has occurred. This allows for leakage of blood into the between injured intima and formation of a hematoma. The hematoma will develop a pseudocapsule, which is able to be ruptured by continuing blood flow through the artery. Because there is no vascular wall structure [7]. Even when a pseudoaneurysm occurred in the MMA passing around the burr hole site due to external ventricular drainage surgery, arterial wall scar eventually became a problem [3]. And in our case, pseudoaneurysm also occurred at the cut end of MMA, which is a bleeding control problem. Nonoperative management of pseudoaneurysms has been suggested; however, such treatment is usually undesirable due to the prolonged duration of many weeks to months, headaches, and the risk rupture [10,13].
Iatrogenic dAVFs are also rare vascular disease entities that consist of direct pathologic connections between meningeal arteries and dural venous sinuses or leptomeningeal veins. Since MMA runs along with middle meningeal vein, surgical manipulation in this area always has the possibility of dural AVF formation. However, The pathophysiology of dAVFs remains to be elucidated and it is of the literatures general opinion that intracranial surgery is able to contributes to the dAVF formation [9]. But most dAVFs are due to trauma, and iatrogenic dAVF formation related to ventriculostomy or burr hole trephination and craniotomy is the level introduced as a case study [15]. Aggressive dAVF with high rupture risk can be predicted when it has cortical reflux, but spontaneous closure occurs in rare low grade dAVF of Cognard classification without cortical venous reflux, and treatment can be surgically treated or more often with embolization as seen in our case more recently [2,5,6].
In other craniotomy with direct dural closure, it will be relatively infrequent, but the dura used in surgery for MMD is to be used for indirect revascularization by cutting the dura broadly and controlling bleeding of cut section less meticulously than general brain surgery, and the tight dural closure of the dura is less emphasized. So, the possibility of pseudoaneurysm formation or dAVF formation are bound to be a sufficient condition. And, the iatrogenic complications such as above that occurred after revascularization surgery bursts well as you can see in the above case, so it must be treated urgently.
Although pseudoaneurysms and dAVFs may occur due to the intracranial surgery, practioners may be aware of the serious consequences after revascularization surgery. Given the nature of revascularization surgery that use antiplatelet, pseudoaneurysms and dAVFs after that surgery would be more likely to have the rupture, compared to those diseases after other intracranial surgery. In addition, the vulnerability of the arterial wall in patient population with MMD also can become a contributing factor of rupture.
Our cases indicate the salient role of implementing timing: Case I had later onset of treatment, which is further associated with being ruptured, whereas Case II had an early onset of treatment, thus the outcome was good. These results underscore the need for early prevention efforts in patients with diseases that could potentially cause bleeding after revascularization.
We might assess the most common hemodynamic complication after this surgery, but we should pay attention to these vasculopathy along with severe consequences, after the revascularization surgery. It is also necessary to take into account the complexity of patients’ condition and tailor scrupulous treatments because of for using antiplatelet.
Pseudoaneurysm formation or dAVF formation after revascularization surgery is an exceptional case. If patients have such complications, practioner should carefully screen the patients by implementing digital subtraction angiogram to identify anatomic features; as well as consider immediate treatment in any way, including embolization or other surgery.
REFERENCES
1. Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of Moyamoya disease in the United States. Stroke. 1998; Jul. 29(7):1347–51.
2. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995; Mar. 194(3):671–80.
3. Grandhi R, Zwagerman NT, Lee P, Jovin T, Okonkwo DO. Iatrogenic pseudoaneurysm of the middle meningeal artery after external ventricular drain placement. J Neuroimaging. 2015; Jan-Feb. 25(1):140–1.
4. Kim H, Jang DK, Han YM, Sung JH, Park IS, Lee KS, et al. Direct bypass versus indirect bypass in adult Moyamoya angiopathy with symptoms or hemodynamic instability: a meta-analysis of comparative studies. World Neurosurg. 2016; Oct. 94:273–84.
5. Komiyama M, Yasui T, Tamura K, Nagata Y, Fu Y, Yagura H. Chronic subdural hematoma associated with middle meningeal arteriovenous fistula treated by a combination of embolization and burr hole drainage. Surg Neurol. 1994; Oct. 42(4):316–9.
6. Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001; May. 22(5):992–6.
7. Madhusudan HV, Krishnamoorthy N, Suresh PK, Subramaniam V. Superficial temporal artery pseudoaneurysm presenting as extradural hematoma: a case report and review of literature. Asian J Neurosurg. 2015; Apr-Jun. 10(2):63–5.
8. Mesiwala AH, Sviri G, Fatemi N, Britz GW, Newell DW. Long-term outcome of superficial temporal artery-middle cerebral artery bypass for patients with Moyamoya disease in the US. Neurosurg Focus. 2008; 24(2):E15.
9. Nabors MW, Azzam CJ, Albanna FJ, Gulya AJ, Davis DO, Kobrine AI. Delayed postoperative dural arteriovenous malformations. Report of two cases. J Neurosurg. 1987; May. 66(5):768–72.
10. Pourdanesh F, Salehian M, Dehghan P, Dehghani N, Dehghani S. Pseudoaneurysm of the superficial temporal artery following penetrating trauma. J Craniofac Surg. 2013; Jul. 24(4):e334–7.
11. Qian C, Yu X, Li J, Chen J, Wang L, Chen G. The efficacy of surgical treatment for the secondary prevention of stroke in symptomatic Moyamoya disease: a meta-analysis. Medicine (Baltimore). 2015; Dec. 94(49):e2218.
12. Sakamoto T, Kawaguchi M, Kurehara K, Kitaguchi K, Furuya H, Karasawa J. Risk factors for neurologic deterioration after revascularization surgery in patients with Moyamoya disease. Anesth Analg. 1997; Nov. 85(5):1060–5.
13. Skaf GS, Domloj NT, Salameh JA, Atiyeh B. Pseudoaneurysm of the superficial temporal artery: a complication of botulinum toxin injection. Aesthetic Plast Surg. 2012; Aug. 36(4):982–5.
14. Sun H, Wilson C, Ozpinar A, Safavi-Abbasi S, Zhao Y, Nakaji P, et al. Perioperative complications and long-term outcomes after bypasses in adults with Moyamoya disease: a systematic review and meta-analysis. World Neurosurg. 2016; Aug. 92:179–88.
15. Vadivelu S, Xin X, Loven T, Restrepo G, Chalif DJ, Setton A. Iatrogenic dural arteriovenous fistula and aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2012; May. 32(5):E1.
16. Yilmaz EY, Pritz MB, Bruno A, Lopez-Yunez A, Biller J. Moyamoya: Indiana University Medical Center experience. Arch Neurol. 2001; Aug. 58(8):1274–8.
Fig. 1.
Pseudoaneurysm at proximal part of anastomosis site of STA. Pseudoaneurysm from middle meningeal artery on digital subtraction angiogram (white arrow heads) (A) anterior-posterior view and (B) lateral view. (C) Brain computed tomography (CT) showed epidural hemorrhage due to ruptured pseudoaneurysm. (D) Brain CT showed parenchymal low density at ipsilateral hemisphere after hematoma evacuation. STA, superficial temporal artery
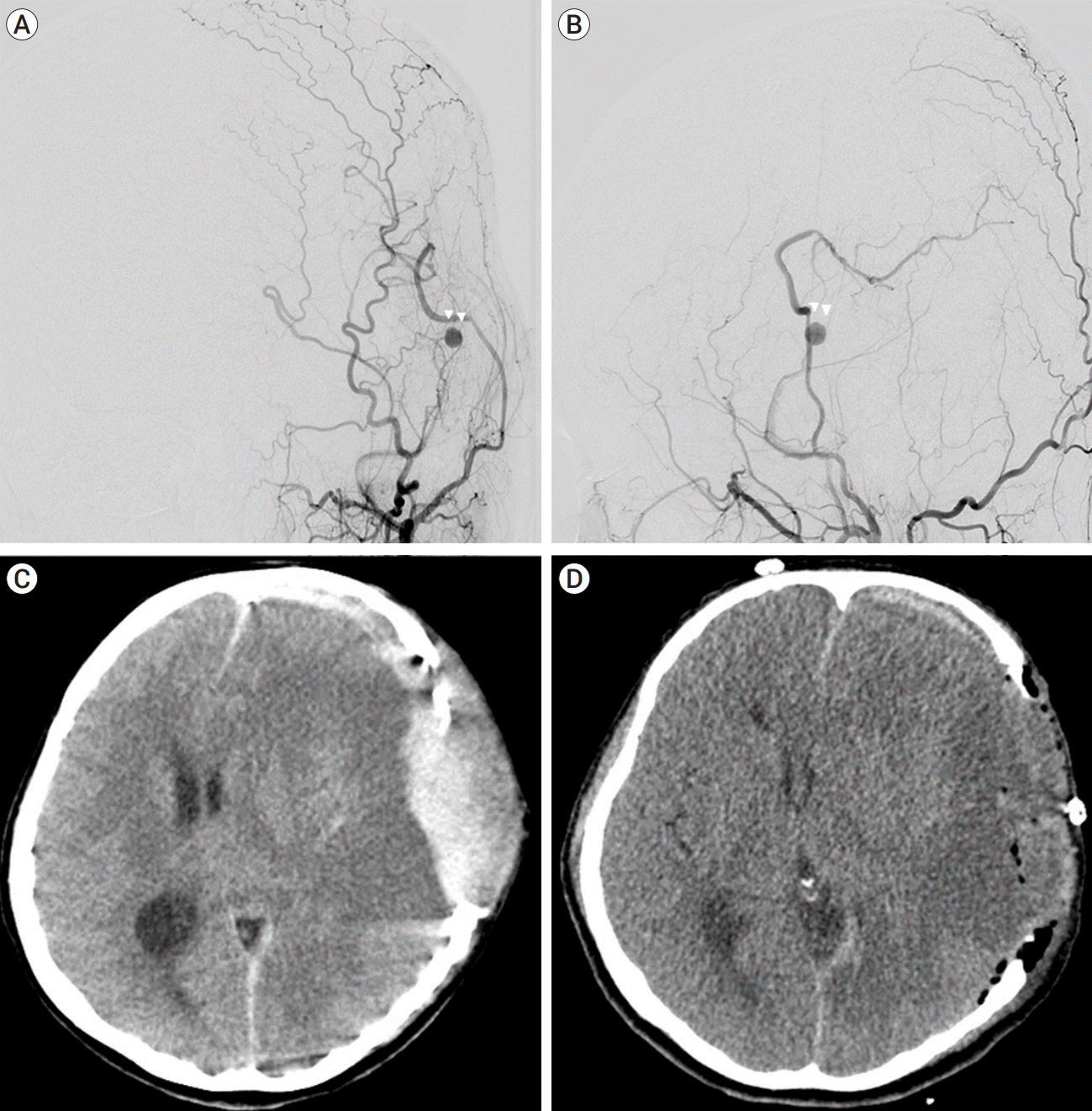
Fig. 2.
dAVF formation at right middle meningeal artery (MMA). dAVF occurring between MMA and middle meningeal vein on digital subtraction angiogram (white arrow heads) (A) anterior-posterior view and (B) lateral view. (C) Occluded dAVF beside black arrows on digital subtraction angiogram after glue embolization. dAVF, dural arteriovenous fistula




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download