Abstract
Objective
Although chronic carotid artery occlusion seems to be associated with significant risk of ischemic stroke, revascularization techniques are neither well established nor widespread. In contrast, extracranial-intracranial bypass is common despite the lack of evidence regarding neurological improvement or prevention of ischemic events. The aim of current review is to evaluate the effectiveness of various methods of recanalization of chronic carotid artery occlusion.
Methods
Comprehensive literature search through PubMed, Scopus, Cochrane and Web of Science databases performed. Various parameters were assessed among patients underwent surgical, endovascular and hybrid recanalization for chronic carotid artery occlusion.
Results
40 publications from 2005 to 2021 with total of more than 1300 cases of revascularization of chronic carotid artery occlusion have been reviewed. Further parameters were assessed among patients underwent surgical, endovascular and hybrid recanalization for chronic carotid artery occlusion: mean age, male to female ratio, mean duration of occlusion before treatment, rate of successful recanalization, frequency of restenosis and reocclusion, prevalence of ischemic stroke postoperatively, neurological or other symptoms improvement and complications. Based on proposed through reviewed literature indications for revascularization and predictive factors of various recanalizing procedures, an algorithm for clinical decision making have been formulated.
Conclusions
Although treatment of chronic carotid artery occlusion remains challenging, current literature suggests revascularization as single option for verified neurological improvement and prevention of ischemic events. Surgical and endovascular procedures should be taken into account when treating patients with symptomatic chronic carotid artery occlusion.
Chronic carotid artery occlusion (CCAO) was reported to be associated with an annual risk of 6% to 20% for ipsilateral recurrent stroke, despite intensive medical management [9,24]. In the presence of substantial contralateral carotid artery stenosis, internal carotid artery (ICA) occlusion doubles the risk for subsequent contralateral stroke [3]. Occlusion of cervical ICA causes chronic hypoperfusion and cerebral ischemia, extracranial-intracranial (EC-IC) bypass surgery for this type of ischemia had been failed to show improvement by the results of cooperative study [20,34,46]. Later studies confirmed inefficiency of EC-IC bypass in prevention of ischemic stroke despite role for revascularization in a highly selected group of patients in whom inadequate hemodynamics can be shown [1,11,14,21,38,40]. According to Cochrane systematic review EC-IC bypass surgery in patients with symptomatic CCAO disease was neither superior nor inferior to medical care alone [10].
For two past decades increasing number of papers suggests advantages of CCAO recanalization compared to medical treatment or bypass surgery. Thus, in contrast to the only subjective improvement in patients who underwent bypass surgery, some studies shown an objective improvement in cognitive functions in patients after CCAO revascularization [8,27,51].
Since first described successful endovascular recanalization of CCAO [45], numerous techniques including surgical, endovascular and hybrid with a wide range of indications and as well complications have been proposed. In this context it became necessary to review heterogenous data for future application to routine clinical practice. The aim of current review is to systematize previously published information on treatment strategies for CCAO and to establish algorithm for clinical decision making based on that data.
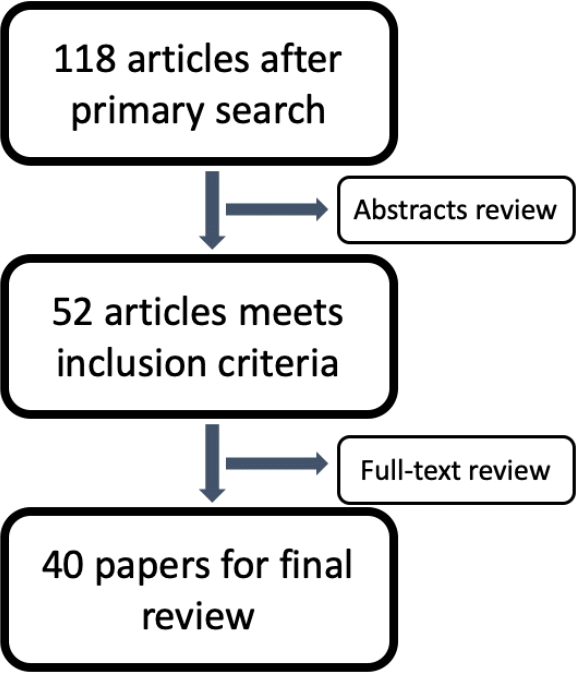
Electronic databases of PubMed, Scopus, Cochrane and Web of Science were searched up for articles describing treatment of CCAO. Full-text articles in English were included (Fig. 1). Analyzed article types were case reports, case series (>5 cases), retrospective and prospective trials, reviews when contained required data. Further parameters were assessed among patients underwent surgical, endovascular and hybrid recanalization for CCAO: mean age, male to female ratio, mean duration of occlusion before treatment, rate of successful recanalization, frequency of restenosis and reocclusion, prevalence of ischemic stroke postoperatively, neurological or other symptoms improvement and complications. Recanalization was recognized as successful with the value of The Thrombolysis in Myocardial Infarction (TIMI) ≥2 and/or Thrombolysis in Cerebral Infarction (TICI) ≥IIb. Clinical improvement suggested with modified Rankin scale dynamic ≥1 postoperatively and/or the absence of transitory ischemic attacks (TIA) or ischemic stroke at 12-month follow-up. SPSS vs. 23 was used for data analysis mostly by descriptive statistics for characterization of numeral data.
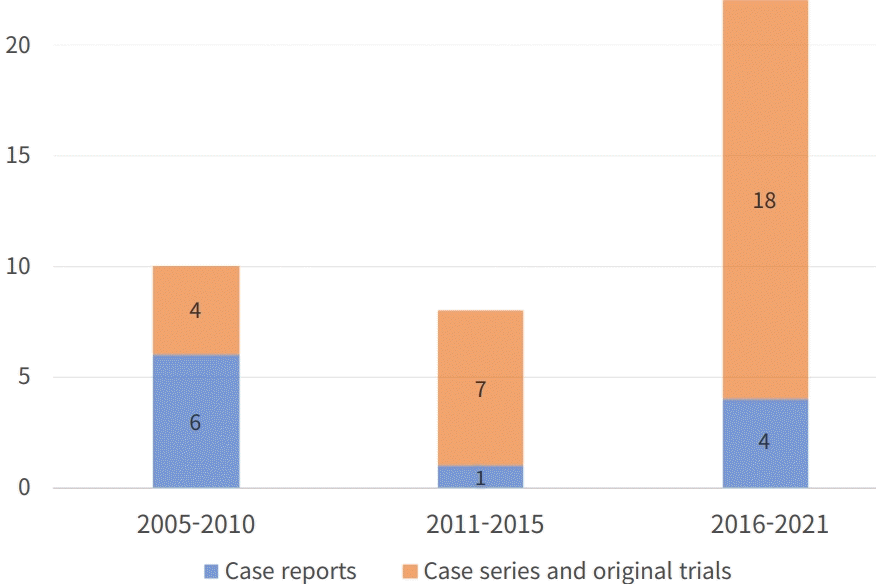
40 publications with total number of 1305 cases, including 11 case reports, 14 case series, 14 original studies (8 retrospective and 6 prospective) and 1 review from 2005 to 2021 matched the criteria with an increasing number of studies in recent 5 years (Fig. 2).
Most papers (28,688 cases) discussing endovascular treatment (ET) of CCAO, 8–hybrid surgery and 4 carotid endarterectomy (CEA). 57 cases of conservative management also presented. There is a discrepancy between the number of patients and the number of cases of recanalization by one method or another in some studies due to the fact that some patients had had occlusions on both sides which were recanalized. Data on studied population, mean duration of occlusion before treatment, rate of successful recanalization, frequency of restenosis and reocclusion, prevalence of ischemic stroke postoperatively and complications summarized in Table 1.
Current literature mainly concerns occlusions caused by atherosclerosis. 34 cases (2.65%) of recanalization of radio induced occlusions presented with one particular case report [32] focused on technique and nuances of endovascular recanalization of radio induced CCAO. It should be noted that successful recanalization rate in this subgroup was lower than the average among studies (63.8 versus 76%) with higher rate of vascular complications (12.25 versus 8%). All published cases of radio induced stenosis were treated by endovascular procedures.
A total of 39 cases (3%) of common carotid artery (CCA) occlusions have been described among reviewed literature revascularized by either surgical or endovascular and hybrid methods. There were no substantial differences in recanalization rates and complications between studies dedicated to ICA and CCA recanalization.
Total improvement of neurological or cognitive functions among patients with successful recanalization vary from 51.3% in ET group to 95.5 in hybrid group with median value of 87%.
Natural history of CCAO hasn’t been well described. Xu et al. [49] determined 4 categories for natural history of CCAO: I - sufficient collateral compensation with no impairment in the cerebrovascular reserve, the recurrence rate for symptoms is 2–8% annually; II - insufficient collateral compensation with damaged cerebrovascular reserve, the risk of ischemic symptoms may be as high as 30% per year; III - effective collateral circulation compensation accompanied by emboli from a narrow common or external carotid artery, emboli from a proximal or distal ICA stump or emboli from a diseased contralateral artery; IV - spontaneous recanalization of carotid artery occlusion (CAO).
Effective leptomeningeal collateral circulation and the presence of more than 2 collaterals have been associated with good clinical condition without severe disability [44].
Previous ischemic event is also a predictive factor of recurrent stroke in patients with ICA occlusion while patients without previous ischemic events are at risk of 0.3% per year in the publication-bias adjusted analysis [15].
Buslovich S et al. [4] reported 3 cases of spontaneous recanalization of CCAOs transformed to high grade (95–99%) stenosis. Mechanisms of such recanalization hadn’t been well established wherein vasa vasorum formation proposed as possible one [4]. In spite of the premise first advocated by Gomensoro et al. that “when a lesion progressed to complete thrombosis, there was no longer any possibility for emboli to pass into the distal circulation” [12], subsequent study of 154 cases of CCAO demonstrated 10.3% of recanalization at a median of 53 month. Furthermore, recanalization leads to significant neurological events by further emboli to the cerebral cortex [31].
Major neurological symptoms among patients with CCAO are: numbness or weakness in face or limbs, aphasia, impaired vision, loss of balance and dizziness and cognitive impairment, verified by standard assessment scales (mini-mental state examination (MMSE), Montreal Cognitive Assessment (MoCA) and others).
Successful recanalization of symptomatic CCAO lowered by about 80% the likelihood of thromboembolisms, compared with medical management [5]. Kao HL et al. [23] reported significant difference in cumulative events of TIA or any stroke or death at 7 years follow-up among recanalized and non-recanalized groups excluding patients with periprocedural events after endovascular procedure with almost 60% reduction of risk in recanalized group.
According to Fan et al. [8] endovascular revascularization is superior to conservative management in improvement of neurological condition and cognitive functions. Prospective studies also revealed improvement in global cognitive function, attention and psychomotor processing speed in patients with CCAO after successful carotid artery stenting [8,27,51].
Hasan D et al. revealed substantial decrees of systolic blood pressure in patients with CCAO after successful recanalization [16]. This unobvious consequence of recanalization is, however, significant in reducing end-organ damage such as retinopathy, left ventricular hypertrophy, impaired renal function and other.
According to Hu Y et al. [18], even the external carotid artery (ECA) recanalization could have significant impact on neurological presentation among patients with chronic CAO.
Few studies described predictive systems for recanalization of CCAO: 1 – for CEA, 4 – for ET and 3 – for hybrid surgery.
Results of retrospective analysis of predictive value of color Doppler flow imaging made by Liu Y et al. [30] suggests length of the occlusion and proximal to distal diameter ratio as criteria for successful CCAO recanalization by CEA.
According to Chen et al., no history of neurologic event, nontapered stump, distal ICA reconstitution by contralateral injection, and distal ICA reconstitution at communicating or ophthalmic segments were identified as independent negative predictors for technical success of ET [6].
Fan et al. [8] described 3 types of CCAO depending on its length with regard to ET: type 1 is located at the cervical segment, easy to manipulate with, a single stent may be sufficient to recanalization; type 2 – occlusion stretches from cervical to the petrous segment, with two or more stents required; type 3 – from C1 to ophthalmic or communicating segment, which is most difficult and carries substantial risk. Thus, recanalization rate of 73% in the patient series of Kao et al. [23] could be explained by inclusion of patients with occlusion at the cavernous to petrous portions of ICA.
As shown by Lee et al. [25], complication rate of endovascular revascularization increased dramatically when occlusion reached clinoid segment of ICA (C5 according to the Bouthillier classification). Among this group it had been higher rates of technical failure (48%), periprocedural complications (22%), and reocclusion (92%), which cancel out the overall benefit of recanalization.
Based on 100 consecutive cases of CAO, Hasan D et al. [16] proposed a radiographic classification of CCAO that could be applied to predict the success of revascularization using endovascular technique. Four types of occlusions were distinguished: type A – occlusion of the cervical ICA is tapered with proximal lumen patency and cavernous and/or petrous segments are reconstructed; type B - occlusion of the cervical ICA is not tapered, immediate proximal portion of ICA lumen is patent and cavernous and/or petrous segments are reconstructed; type C – occlusion is at the CCA bifurcation, cavernous and/or petrous segments are reconstructed; type D is the same as type C, except no reconstruction of the cavernous and/or petrous segments. Application of proposed classification to clinical practice revealed recanalization success rate of 100% in types A and B, and much more challenging procedures in types C and D with success rates of 50 and 25% respectively and higher complication rate [16].
Zanaty M et al. reported successful and complications free recanalization of Hasan type C occlusions via hybrid surgery and proposed bypass surgery for type D patients [51].
As shown by Liu B et al. [29], hybrid recanalization would be more likely to be unsuccessful if the ICA was occluded at the ophthalmic or supraclinoid segment and the thrombus lesion extended to the cervical segment. Possible markers of plaque localization in supraclinoid segment are absence of collateral blood flow through ophthalmic anastomosis and anastomotic blood flow from the contralateral carotid artery through the anterior communicating artery or from the vertebrobasilar artery through the posterior communicating artery.
High occlusions reported to be hardly recanalized either by hybrid procedures. The success rates of distal ICA recanalization at the petrous segment or below, cavernous segment, and clinoid segment or above were 100, near 30, and less than 15%, respectively in major case series [50]. The results demonstrated that the level of distal ICA reconstitution was the only independent factor affecting the success of hybrid surgery [50].
According to Li J et al. [26] the technical success rate of recanalization of nontaper or nonstump chronic ICA occlusions with hybrid technique is better than that with endovascular interventions, with fewer perioperative complications.
Despite some studies revealed dependence of recanalization rate on duration of occlusion [50], further research needed to determine the optimal time for recanalization.
5 studies described results of surgical CCAO revascularization by CEA with 4 focused on CEA as the only treatment option and 1compared to ET and hybrid surgery. There are 1 systematic review, 2 case series and 2 trials (1 retrospective and 1 prospective). Most presented cases concern atherosclerotic ICA occlusions while 29 cases (8.4%) of CCA occlusions were also described. High recanalization rate (91%) seems to be associated with rigorous selection of patients underwent CEA: only patients with short-length occlusions were considered candidates for surgery. Thus, CEA was shown to be an alternative to ET in cases of segmental extracranial ICA occlusions [2]. Two important prerequisites for successful segmental ICA occlusion CEA were proposed: visualization of distal ICA and fully patent and functional circle of Willis [2].
ET remains most studied method for recanalization of CCAO with more than 600 cases described in literature.
Endovascular recanalization compares favorably with surgical treatment by using local anesthesia and minimal invasiveness [45], although some authors also suggests regional cervical block for CEA [35]. Another advantage is that it’s application is not limited by extracranial portion of ICA.
Numerous technical equipment presented for CCAO ET, including guidewires, microcatheters, balloons, stents and emboli-protecting devices. Principle scheme of endovascular recanalization comprises: plaque penetration by guidewire and passage of microcatheter, balloon angioplasty and telescopic stenting of affected segment and aspiration of thrombi and atheromatous masses. Prevention of distal emboly usually achieved by preceding balloon occlusion of ECA and CCA while some authors prefer reversion of blood-flow from CCA to femoral vein. Usage of distal emboly protection devices is mandatory but often restricted by ICA diameter distal to occlusion. According to Lin et al. using of distal protection systems available in 73% of cases [28].
Most common complications of ET are (in descending order): distal emboly, perforation of ICA, dissection, bradycardia with hypotension and carotid-cavernous fistula (CCF). Current literature presented with 1 case of dissection hindering revascularization, 2 lethal subarachnoid hemorrhage and 2 lethal intracerebral hemorrhage. Asymptomatic dissections, perforations and so-called minor complications are much more common.
Few tips proposed to decrease complications rate of endovascular procedures. In particular, Rostambeigi et al. advocated using of ultrasound guidance to improve the outcome of the endovascular procedures in high-risk patients with carotid occlusion [36]. Nico et al. [32] suggests using of echographic control to avoid the subintimal progression of the guidewire while entering the internal carotid at the end of the common carotid artery. Additionally, uncommon technique of a long dilatation balloon had been proposed for less traumatic, homogeneous dilatation of the entire segment.
Although complicated cases of CCAO could be challenging for ET, well-trained specialists have wide opportunities for interventions. Thus, extraordinary experience of retrograde recanalization of chronic ICA occlusion presented by Uno T et al. [48]. Transradial approach had been used for passing the occlusion site by guidewire from distal to proximal direction via vertebral and posterior communicating arteries, which allowed to complete the endovascular embolization attempt initially undertaken by the femoral approach.
Determination of true lumen of the ICA and traversing a long occlusive lesion without incurring additional arterial injury remains challenging regarding endovascular recanalization of CCAO [28,36]. Therefore, Shih YT et al. [41] introduced hybrid technique for recanalization of CCAO with more success and fewer complication rate. Main advantage of hybrid revascularization is the possibility for reconstruction of proximal ICA via standard CEA with ongoing application of endovascular technique for opening of inaccessible for direct surgery regions.
Jiang et al. [22] reported successful recanalization of 83.3% of CCAO using combined (endovascular procedure + CEA) approach rather than 35.7% from the initial ET or CEA along. 75 and 69% successful recanalization were seen in type C and D occlusions respectively. The cumulative probability of stroke and death within 30 days plus ischemic ipsilateral stroke beyond 30 days at 6 months and 1 year of 5.1% was much lower than 21% of the surgical arm, and 22.7% of the medical arm in the carotid occlusion surgery study (COSS) trial [46].
Overall complication rate among reviewed studies were 8% including dissections both symptomatic and clinically silent, CCFs, intraoperative distal emboly, ICA perforations, hyperperfusion syndrome and bradycardia with hypotension. Mean declared rate of vascular complication was 8.4% (range from 0 to 40%) and related to studies on ET. Few complications of CEA and hybrid surgery reported. Most of them were asymptomatic hyperperfusion, neck hematoma and recurrent laryngeal nerve injury.
Few authors described new bright spots on diffusionweighted imagings (DWIs) after endovascular CCAO revascularization, most of them become clinically silent [42].
Most cases of total ICA occlusion characterized by hemodynamic compromise which according to Ogasawara and colleagues was the strong predictor of postoperative hyperperfusion syndrome [33]. Transcranial doppler intraoperatively and daily after intervention with evaluation of blood-flow velocity of medial cerebral artery (MCA) is essential for earlier revealing of hyperperfusion syndrome. Diagnostic criterion for hyperperfusion syndrome is twofold and more increase in the MCA mean flow velocity after revascularization of the internal carotid artery [39].
Frequent complication of CCAO recanalization is carotid-cavernous fistula formation. CCF may occur in 4% of endovascular CCAO recanalization. According to Chen et al., female gender and carotid reconstitution distal to ophthalmic segment both predicts CCF occurrence [6]. Iatrogenic CCF in this situation is usually self-limited with benign clinical course.
According to Hudson et al., balloon angioplasty of the proximal cervical ICA is strongly associate with periprocedural bradycardia (including asystole and symptomatic cases), while no incidence of bradycardia was seen during/after hybrid recanalization [19].
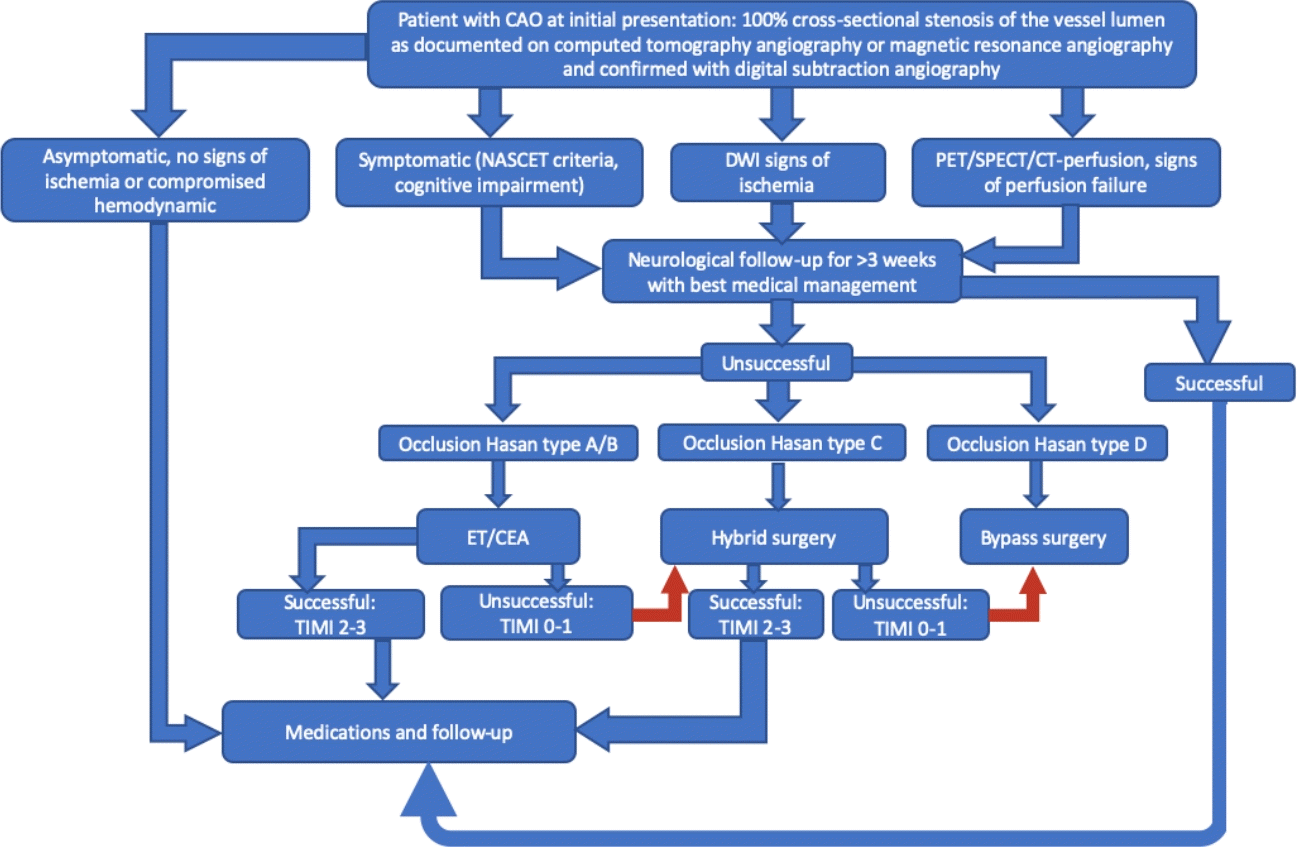
Based on proposed through reviewed literature indications for revascularization and predictive factors of various recanalizing procedures, an algorithm for clinical decision making have been formulated (Fig. 3). According it symptomatic cases of CCAO should be treated conservatively for 3 weeks. If second assessment reveal symptoms of TIA through 3 weeks period, persistent neurological deficit or cognitive impairment, it should be considered for revascularization procedure. The final choice of procedure should be based on type of occlusion by Hasan [51], assessed through computed tomography angiography (CTA). Hasan type A and B occlusions are characterizing by favorable outcome and could be recanalized by CEA or endovascular procedure. Revascularization of Hasan type C occlusions is much more challenging and requires application of hybrid technique. Hasan type D occlusions, according to literature, has few perspectives for successful revascularization and should be considered for bypass surgery or further conservative treatment. Asymptomatic cases of CCAO as well as successfully recanalized should underwent continuous medical therapy and thorough follow-up, including neurological examination, cognitive assessment, color Doppler flow imaging, CTA and assessment of cerebral perfusion.
Significant risks of complications and further neurological deterioration should be taken into account and discussed before procedure. Nonetheless, symptomatic patients with Hasan type A, B and C occlusions who didn’t succeed with best medical management should be considered as candidates for revascularization.
The mechanism of stroke in patients with CCAO occlusions could be related to violation of microcirculation due to hemodynamic compromise and inadequate cerebral perfusion via collateral vessels [23]. Two types of hemodynamic failure had been described among patients with CCAO. Type I associated with lack of hemodynamic reserve, which could be assessed by blood flow studies, including computed tomography perfusion; magnetic resonance imaging perfusion, Xenon CT and single photon emission computed tomography (SPECT) combined with vasodilator challenge (acetazolamide or hypercapnia). Type II hemodynamic failure is collapse of vascular reserve in combination with increased oxygen extraction fraction measured by PET. This type of failure is much more complex for assessment due to requirement of cyclotron to generate O15. As shown by Grubb et al. [13], up to 28.2% of patients with CCAO in association with type II hemodynamic failure could be at risk of ipsilateral stroke over a mean follow-up period of 31.5 month. It’s much exceed 4.7% risk in patients with type I failure. Based on these data, the indication for revascularization in asymptomatic patients with CCAO and type I hemodynamic failure is questionable [17]. If ipsilateral-to-contralateral oxygen extraction fracture ratio is greater than 1.13 or symptomatic patients with stage I or II hemodynamic failure, surgical treatment is usually recommended [50].
Reversal of ophthalmic artery flow direction has been shown as another good marker of hemodynamic insufficiency caused by proximal ICA occlusion [37]. According to Kao et al., it takes place in 87% of patients with CCAO and resolved after successful recanalization in 80% of cases [23]. Furthermore, failure to flow normalization was found to be a good marker for in-stent reocclusion. Therefore, duplex ophthalmic flow analyzes is important for follow-up evaluation of patients after endovascular CAO recanalization.
Earlier attempts of surgical revascularization by CEA shown significant failure rate and risk [7,47]. Thompson et al. reported the results of CEA in 118 patients with chronic CAO [47]. Recanalization was achieved in 41% with a mortality of 6.2%. First surgical recanalization through CEA with additional use of Fogarty balloon-catheter was performed Shucart and Garrido in 1976 [43].
Previous studies showed effectiveness of recanalization of chronically occluded subclavian and iliac arteries. Furthermore, regarding hypothesis of Terada et al., rate of restenosis depends on diameter of the native artery: if it’s grater then 3 mm, the restenosis rate will be low. Therefore, the restenosis rate can be supposed to be low in the totally occluded cervical ICA [45]. Before 2005 endovascular recanalization of the totally occluded cervical ICA in chronic stage hadn’t been reported because of the risk of embolic stroke related to the recanalized artery [45], although it should be noted, that according report of Lin et al. [28], first patients were treated since October 2002. Since first described successful endovascular recanalization of chronically occluded ICA by Terada et al. in 2005, to date more than 1200 patients had undergone this procedure according literature.
Indications for revascularization of chronically occluded cervical ICA are ineffectiveness of antiplatelet therapy in preventing TIA and hemodynamic cerebral ischemia proven by SPECT or PET scanning results [17,45]. Moreover, retrograde filling is an important criterion for recanalization treatment of CCAO [49].
In case of CCAO, the complication rate indicates less morbidity then the natural history of the disease and this combined with dramatic improvement noted in patients with successful recanalization can justify and encourage the procedure’s use [16].
Although treatment of chronic carotid artery occlusion remains challenging, current literature suggests revascularization as single option for verified neurological improvement and prevention of ischemic events. Surgical and endovascular procedures should be taken into account when treating patients with symptomatic chronic carotid artery occlusion.
This study contains many limitations. Heterogeneity of published data doesn’t allow accurate comparison of surgical, endovascular and hybrid methods with precise identification of parameters of interest. Never the less, authors believe the work done is useful for current clinical practice and future researches.
REFERENCES
1. Adams HP Jr, Power WJ, Grubb RL Jr, Clarke WR, Woolson RF. Preview of a new trial of extracranial-to-intracranial arterial anastomosis: the carotid occlusion surgery study. Neurosurg Clin N Am. 2001; Jul. 12(3):613–24. ix–x.
2. Babić S, Tanasković S, Nešković M, Gajin P, Nenezić D, Stevanović P, et al. Surgical treatment of proximal segmental occlusion of the internal carotid artery. Surg Res Pract. 2019; Jan. 2019:2976091.
3. Barnett HJ. Status report on the North American symptomatic carotid surgery trial. J Mal Vasc. 1993; 18(3):202–8.
4. Buslovich S, Hines GL. Spontaneous recanalization of chronic internal carotid artery occlusions: report of 3 cases. Vasc Endovascular Surg. 2011; Jan. 45(1):93–7.
5. Cagnazzo F, Lefevre PH, Derraz I, Dargazanli C, Gascou G, Riquelme C, et al. Endovascular recanalization of chronically occluded internal carotid artery. J Neurointerv Surg. 2020; Oct. 12(10):946–51.
6. Chen YH, Leong WS, Lin MS, Huang CC, Hung CS, Li HY, et al. Predictors for successful endovascular intervention in chronic carotid artery total occlusion. JACC Cardiovasc Interv. 2016; Sep. 9(17):1825–32.
7. De Bakey ME, Crawford ES, Cooley DA, Morris GC Jr, Edward Garret H, Fields WS. Cerebral arterial insufficiency: one to 11-year results following arterial reconstructive operation. Ann Surg. 1965; Jun. 161(6):921–45.
8. Fan YL, Wan JQ, Zhou ZW, Chen L, Wang Y, Yao Q, et al. Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion: a prospective, controlled, single-center study. Vasc Endovascular Surg. 2014; May. 48(4):305–10.
9. Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD Jr. Population-based study of symptomatic internal carotid artery occlusion: Incidence and long-term follow-up. Stroke. 2004; Aug. 35(8):e349–52.
10. Fluri F, Engelter S, Lyrer P. Extracranial‐intracranial arterial bypass surgery for occlusive carotid artery disease. Cochrane Database Syst Rev. 2010; Feb. 17. 2010(2):CD005953.
11. Galkin PV, Gushcha AO, Antonov GI. Surgical treatment of the internal carotid artery atherosclerotic occlusion. Zh Nevrol Psikhiatr Im S S Korsakova. 2014; 114(7):67–72.
12. Gomensoro JB. Joint study of extracranial arterial occlusion: VIII. Clinical-radiographic correlation of carotid bifurcation lesions in 177 patients with transient cerebral ischaemic attacks. JAMA. 1973; May. 224(7):985–91.
13. Grubb RL Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998; 280(12):1055–60.
14. Grubb RL Jr, Powers WJ, Clarke WR, Videen TO, Adams HP Jr, Derdeyn CP, et al. Surgical results of the carotid occlusion surgery study. J Neurosurg. 2013; Jan. 118(1):25–33.
15. Hackam DG. Prognosis of asymptomatic carotid artery occlusion: systematic review and meta-analysis. Stroke. 2016; May. 47(5):1253–7.
16. Hasan D, Zanaty M, Starke RM, Atallah E, Chalouhi N, Jabbour P, et al. Feasibility, safety, and changes in systolic blood pressure associated with endovascular revascularization of symptomatic and chronically occluded cervical internal carotid artery using a newly suggested radiographic classification of chronically occluded cervical internal carotid artery: pilot study. J Neurosurg. 2018; May. 1–10.
17. Hauck EF, Ogilvy CS, Siddiqui AH, Hopkins LN, Levy EI. Direct endovascular recanalization of chronic carotid occlusion: should we do it? Case report. Neurosurgery. 2010; Oct. 67(4):E1152–9. discussion E1159.
18. Hu Y, Qian H, Shi X. Treatment of chronic internal carotid artery occlusion by ipsilateral external carotid endarterectomy. Br J Neurosurg. 2020; Feb. 1–3.
19. Hudson JS, Zanaty M, Wadman V, Nakagawa D, Ishii D, Roa JA, et al. Bradycardia and asystole in patients undergoing symptomatic chronically occluded internal carotid artery recanalization. World Neurosurg. 2019; Nov. 131:e211–7.
20. Ishikawa T, Kuroda S, Hokin K, Kamiyama H, Abe H. Can EC-IC bypass prevent brain ischemia from recurring? No Shinkei Geka. 1998; Sep. 26(9):823–9.
21. JET Study Group. Japanese EC-IC bypass trial (JET study): study design and interim analysis. Surg Cereb Stroke. 2002; 30(2):97–100.
22. Jiang WJ, Liu AF, Yu W, Qiu HC, Zhang YQ, Liu F, et al. Outcomes of multimodality in situ recanalization in hybrid operating room (MIRHOR) for symptomatic chronic internal carotid artery occlusions. J Neurointerv Surg. 2019; Aug. 11(8):825–32.
23. Kao HL, Lin MS, Wang CS, Lin YH, Lin LC, Chao CL, et al. Feasibility of endovascular recanalization for symptomatic cervical internal carotid artery occlusion. J Am Coll Cardiol. 2007; Feb. 49(7):765–71.
24. Klijn CJ, van Buren PA, Kappelle LJ, Tulleken CA, Eikelboom BC, Algra A, et al. Outcome in patients with symptomatic occlusion of the internal carotid artery. Eur J Vasc Endovasc Surg. 2000; Jun. 19(6):579–86.
25. Lee CW, Lin YH, Liu HM, Wang YF, Chen YF, Wang JL. Predicting procedure successful rate and 1-year patency after endovascular recanalization for chronic carotid artery occlusion by CT angiography. Int J Cardiol. 2016; Oct. 221:772–6.
26. Li J, Wang C, Zou S, Liu Y, Qu L. Hybrid surgery for nontaper or nonstump lesions in symptomatic subacute or chronic internal carotid occlusion: a better solution. World Neurosurg. 2019; Feb. 122:e1416–25.
27. Lin MS, Chiu MJ, Wu YW, Huang CC, Chao CC, Chen YH, et al. Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion and cerebral ischemia. Stroke. 2011; Oct. 42(10):2850–4.
28. Lin MS, Lin LC, Li HY, Lin CH, Chao CC, Hsu CN, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Interv. 2008; Oct. 1(2):119–25.
29. Liu B, Wei W, Wang Y, Yang X, Yue S, Zhang J. Estimation and recanalization of chronic occluded internal carotid artery: hybrid operation by carotid endarterectomy and endovascular angioplasty. World Neurosurg. 2018; Dec. 120:e457–65.
30. Liu Y, Jia L, Liu B, Meng X, Yang J, Li J, et al. Evaluation of endarterectomy recanalization under ultrasound guidance in symptomatic patients with carotid artery occlusion. PLoS One. 2015; Dec. 10(12):e0144381.
31. Morris-Stiff G, Teli M, Khan PY, Ogunbiyi SO, Champ CS, Hibberd R, et al. Internal carotid artery occlusion: its natural history including recanalization and subsequent neurological events. Vasc Endovascular Surg. 2013; Nov. 47(8):603–7.
32. Nico L, Cester G, Viaro F, Baracchini C, Causin F. Endovascular recanalization of the common carotid artery in a patient with radio induced chronic occlusion. BMJ Case Rep. 2016; Nov. 2016:bcr2016012722.
33. Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using singlephoton emission computerized tomography scanning. J Neurosurg. 2003; Sep. 99(3):504–10.
34. Ohki T, Parodi J, Veith FJ, Bates M, Bade M, Chang D, et al. Efficacy of a proximal occlusion catheter with reversal of flow in the prevention of embolic events during carotid artery stenting: an experimental analysis. J Vasc Surg. 2001; Mar. 33(3):504–9.
35. Paty PSK, Adeniyi JA, Mehta M, Darling RC III, Chang BB, Kreienburg PB, et al. Surgical treatment of internal carotid artery occlusion. J Vasc Surg. 2003; Apr. 37(4):785–8.
36. Rostambeigi N, Khatri R, Hassan AE, Qureshi AI. Duplex ultrasound assisted endovascular revascularization of chronic internal carotid artery occlusion: technical note. J Vasc Interv Neurol. 2013; Dec. 6(2):42–6.
37. Rutgers DR, Klijn CJ, Kappelle LJ, van Huffelen AC, van der Grond J. A longitudinal study of collateral flow patterns in the circle of Willis and the ophthalmic artery in patients with a symptomatic internal carotid artery occlusion. Stroke. 2000; Aug. 31(8):1913–20.
38. Sasoh M, Ogasawara K, Kuroda K, Okuguchi T, Terasaki K, Yamadate K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003; Jun. 59(6):455–60. discussion 460.
39. Schaafsma A, Veen L, Vos JP. Three cases of hyperperfusion syndrome identified by daily transcranial Doppler investigation after carotid surgery. Eur J Vasc Endovasc Surg. 2002; Jan. 23(1):17–22.
40. Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einhupl K. Improvement of cerebrovascular reserve capacity by ECIC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg. 1994; Aug. 81(2):236–44.
41. Shih YT, Chen WH, Lee WL, Lee HT, Shen CC, Tsuei YS. Hybrid surgery for symptomatic chronic total occlusion of carotid artery: a technical note. Neurosurgery. 2013; Sep. 73(1 Suppl Operative):onsE117–23. discussion onsE123.
42. Shojima M, Nemoto S, Morita A, Miyata T, Namba K, Tanaka Y, et al. Protected endovascular revascularization of subacute and chronic total occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2010; Mar. 31(3):481–6.
43. Shucart WA, Garrido E. Reopening some occluded carotid arteries. Report of four cases. J Neurosurg. 1976; Oct. 45(4):442–6.
44. Sundaram S, Kannoth S, Thomas B, Sarma PS, Sylaja PN. Collateral assessment by CT angiography as a predictor of outcome in symptomatic cervical internal carotid artery occlusion. AJNR Am J Neuroradiol. 2017; Jan. 38(1):52–7.
45. Terada T, Yamaga H, Tsumoto T, Masuo O, Itakura T. 2005. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage. J Neurosurg. 2005; Mar. 102(3):558–64.
46. The EC-IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985; Nov. 7. 313(19):1191–200.
47. Thompson JE, Austin DJ, Patman RD. Carotid endarterectomy for cerebrovascular insufficiency: long-term results in 592 patients followed up to thirteen years. Ann Surg. 1970; Oct. 172(4):663–79.
48. Uno T, Shojima M, Oyama Y, Yamane F, Matsuno A. Retrograde endovascular revascularization for chronic total occlusion of the internal carotid artery: a case report. Acta Neurochir (Wien). 2022; Apr. 164(4):1015–9.
49. Xu B, Li C, Guo Y, Xu K, Yang Y, Yu J. Current understanding of chronic total occlusion of the internal carotid artery. Biomed Rep. 2018; Feb. 8(2):117–25.
50. Yang Y, Liu X, Wang R, Zhang Y, Zhang D, Zhao J. A treatment option for symptomatic chronic complete internal carotid artery occlusion: hybrid surgery. Front Neurosci. 2020; Apr. 14:392.
51. Zanaty M, Howard S, Roa JA, Alvarez CM, Kung DK, McCarthy DJ, et al. Cognitive and cerebral hemodynamic effects of endovascular recanalization of chronically occluded cervical internal carotid artery: single-center study and review of the literature. J Neurosurg. 2019; Mar. 132(4):1158–66.
Table 1.
General characteristics of the studies included in the review
One prospective trial corresponds both CEA and endovascular and hybrid groups concern to hybrid column on the basis of maximal patients treated by hybrid surgery. CEA, carotid endarterectomy; ICA, internal carotid artery; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; CCF, carotid-cavernous fistula




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download