This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
The aim of this study was to evaluate the performance of a clinical decision support system (CDSS) for therapeutic plans in geriatric dentistry. The information that needs to be considered in a therapeutic plan includes not only the patient’s oral health status obtained from an oral examination, but also other related factors such as underlying diseases, socioeconomic characteristics, and functional dependency.
Methods
A Bayesian network (BN) was used as a framework to construct a model of contributing factors and their causal relationships based on clinical knowledge and data. The faster R-CNN (regional convolutional neural network) algorithm was used to detect oral health status, which was part of the BN structure. The study was conducted using retrospective data from 400 patients receiving geriatric dental care at a university hospital between January 2020 and June 2021.
Results
The model showed an F1-score of 89.31%, precision of 86.69%, and recall of 82.14% for the detection of periodontally compromised teeth. A receiver operating characteristic curve analysis showed that the BN model was highly accurate for recommending therapeutic plans (area under the curve = 0.902). The model performance was compared to that of experts in geriatric dentistry, and the experts and the system strongly agreed on the recommended therapeutic plans (kappa value = 0.905).
Conclusions
This research was the first phase of the development of a CDSS to recommend geriatric dental treatment. The proposed system, when integrated into the clinical workflow, is expected to provide general practitioners with expert-level decision support in geriatric dental care.
Go to :

Keywords: Deep Learning, Machine Learning, Geriatrics, Dentists, Decision Making
I. Introduction
Aging involves declining physical function due to the accumulation of damaged tissue and substances in internal and extrinsic systems over time [
1]. The oral health status of older people is characterized by a high level of xerostomia, poor oral hygiene, a high prevalence of periodontitis and dental caries, tooth loss, and oral lesions. Extensive tooth loss affects chewing performance, leading to poor nutrition, weight loss, and impaired communication [
2]. Poor oral health and the presence of underlying diseases are interrelated. Clinical associations have been documented between severe periodontal disease and diabetes mellitus [
3] and ischemic heart disease [
4]. The information that needs to be considered in a therapeutic plan must include not only the oral health status obtained from an oral examination, but also other related factors such as underlying diseases, socioeconomic characteristics, and functional dependency. Treatment options can be altered to accommodate patients with different degrees of dependency, and treatment recommendations for elderly patients must be comprehensive and holistic [
5]. However, there is no gold standard, even among experts, for preparing treatment plans in this complex context.
With advances in digital technology and artificial intelligence, the ability to provide clinicians with clinical decision support has led to impressive new functionality. Artificial intelligence and machine learning programs have shown promising results in data and image interpretation [
6]. A convolutional neural network (CNN) is a type of machine learning that captures patterns from input data in order to predict data output. Studies using CNN algorithms have demonstrated their ability to accurately classify and detect clinical lesions. For example, a faster regional-CNN (faster R-CNN) model was highly accurate in classifying and detecting oral squamous cell carcinoma in photographic images [
7]. The credibility and adoption of clinical decision support systems (CDSSs) depend both on their diagnostic performance and their design for direct interactive use by clinicians. Certain features need to be included in a CDSS if it is to be recognized and integrated into routine workflows [
8]. In particular, a CDSS must be transparent, so that users can understand the rationale behind recommendations. Black boxes are unacceptable. Furthermore, knowledge and information must be delivered in a useful manner. A CDSS should provide guidance in a way that takes users’ knowledge into consideration. The system must be implemented in a way that assists humans, instead of attempting to replace human expertise.
The aim of this study was to evaluate the performance of a CDSS for therapeutic plans in geriatric dentistry. A Bayesian network (BN) was used as a framework to construct a model of contributing factors and their causal relationships based on clinical knowledge and data. The most efficiently performing recent CNN-based algorithm was used to detect oral health status, which was part of the BN structure. The model performance was compared to that of experts in geriatric dentistry. The proposed CDSS, when integrated into the clinical workflow, is expected to provide general practitioners with expert-level decision support in geriatric dental care.
Go to :

II. Methods
The study was conducted using retrospective data extracted from 400 anonymized patients receiving geriatric dental care at the authors’ affiliated university hospital between January 2020 and June 2021. The human research ethics board of Srinakharinwirot University approved the study (IRB No. DENTSWU-EC 16/2561). The requirement for informed consent was waived because anonymized data were used to develop the decision support model. All methods were performed according to standard practice guidelines. The modeling procedure started with the selection of variables to be included in the CDSS’s treatment recommendation algorithms. The author referred to articles and clinical guidelines in gerontology, enhanced by the authors’ experience gained through practice and training, to create a list of associated variables. With the labeled variables, one data element corresponded to an individual patient.
1. Bayesian Network
Bayesian probabilistic models have been used as the foundation for treatment recommendation model. Probabilistic graphical models of BNs are used to represent the uncertainty of domain knowledge for various problems. Conditional probabilities computed from BNs can be used for recommendations in decision support systems. Because of their ability to model uncertainty and causality across variables, BNs are a powerful platform in many therapeutic settings. In a BN, the individual variables are modeled as causal relationships with nodes. A conditional probability table for each node represents the probability of each value for that node. There are two kinds of learning in building a BN. In structural learning, the model learns dependencies between the variables that lie in the data. Parametric learning fills in the parameters (conditional probability tables) describing the strength of the dependencies in the learned structure. In this work, the structure of the BN was created with the help of an experienced clinician. The conditional probabilities of the BN were computed from the training dataset using an expectation-maximization (EM) algorithm [
9]. An EM algorithm is used to find maximum likelihood estimates of parameters in a BN model. EM involves an alternation between performing an expectation (E) step, which computes an expected likelihood by including the latent variables as if they were observed, and a maximization (M) step, which computes the maximum likelihood estimates of the parameters by maximizing the expected likelihood found in the E step. The parameters found in the M step are then used to begin another E step, and the process is repeated. The database contained 24 variables related to the status and interventions for geriatric dental care (
Table 1). The dataset was randomly split into a training dataset (80%, n = 320) and a test dataset (20%, n = 80).
Table 1
Variables and states for geriatric dental care
|
Variable |
State |
|
Sex |
Male, Female |
|
Age (yr) |
60–69, 70–79, above 80 |
|
Socio-economic |
Good, Fair, Poor |
|
Function |
Independent, Semi-dependent, Dependent |
|
Smoking |
No, Yes |
|
Drinking |
No, Yes |
|
Medical history |
No, Yes |
|
Medication |
No, Yes |
|
Oral hygiene |
Good, Fair, Poor |
|
Diet |
Normal, Hard or Sour |
|
Dry mouth |
No, Yes |
|
Chief complaint |
No, Yes |
|
Caries |
No, Yes |
|
Caries expose pulp |
No, Yes |
|
Retained root |
No, Yes |
|
Periodontium |
Within normal limit, Gingivitis, Periodontitis |
|
Reduced posterior support |
No, Yes |
|
Systemic disease treatment |
No, Yes |
|
Prevention |
No, Yes |
|
Chief complaint treatment |
No, Yes |
|
Periodontal treatment |
No, Yes |
|
Operative treatment |
No, Yes |
|
Oral surgery |
No, Yes |
|
Prosthodontic treatment |
No, Yes |

2. Convolutional Neural Network
The oral health status of periodontitis (
Table 1) was determined by applying a CNN to detect periodontally compromised teeth on digital panoramic radiography. The detection of abnormalities in clinical imaging is an important factor related to a successful diagnosis of the disease. CNN-based algorithms for object detection have proven to be effective in identifying anomalies in images. In this research, faster R-CNN was adopted to detect periodontally compromised teeth on digital panoramic radiographs. The faster R-CNN algorithm uses the convolutional features of fast R-CNN and region proposal networks and combines them into a single real-time object detection network [
10]. The object detection experiment used an annotated radiographic image showing the location of periodontally compromised teeth. The images and annotations were used for the purpose of training. Using the Keras ImageDataGenerator (open-source software), images were preprocessed via augmentation. An input image was rescaled to 256 × 256 pixels by the framework and passed to the neural network. The training was run on a system with two GPUs: TitanXP 12 GB, NVIDIA driver v450.102 and CUDA v11.0 (NVIDIA Corporation, Santa Clara, CA, USA). The faster R-CNN architecture was pretrained with COCO detection, and trained using stochastic gradient descent. This study adjusted several hyperparameters to maximize the training performance. The models were trained with 1,882 epochs and 20,000 iterations with a 128 batch size per image.
3. Evaluation
This study applied indicators used to evaluate machine learning algorithms in bioinformatics [
11]. The faster R-CNN model’s performance in detecting periodontally comprised teeth on digital radiographs was evaluated using the precision, recall, F1-score, and area under the precision-recall curve. The validity of the BN model was determined using the area under the receiver operating characteristic curve (AUC). The performance of the treatment suggestion model was compared to that of two board-certified dentists with at least 5 years of experience in geriatric dentistry using an external dataset with known treatment results. Twenty randomly selected data elements from the test dataset (80 data elements) were used to test the performance of the model compared to that of the board-certified dentists. They were each asked to study the electronic health records of each participant and recommend a treatment plan. The kappa statistic was used to determine the statistical significance of the agreement between the dentists’ results and those of the treatment suggestion system. To evaluate the agreement among dentists, the Kendall W coefficient of concordance was used. SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used to perform the data analysis.
Go to :

III. Results
The faster R-CNN model for detecting periodontally compromised teeth was evaluated with the test set. The detection model obtained a precision of 86.69%, a recall of 82.14%, and an F1-score of 89.31%. The area under the precision-recall curve (0.89) showed high accuracy. The output generated by the object detection model is shown in
Figure 1. All periodontally compromised teeth were correctly detected by the faster R-CNN model.
Figure 2 shows the structure of the BN, and also the BN’s conditional probability distribution, which was constructed using one training data set. A conditional probability table (CPT) specifying the probabilistic correlations between the variables is presented for each node. Depending on whether a parent node is observed, the CPT specifies the conditional likelihood of a child node. The normalized conditional probabilities that are compatible with the states and conditions defined on the evidence are displayed in the node bar. The CPT is normalized so that the maximum value is 1. It is not possible to assess a patient’s situation by assigning the most probable values individually to each variable due to the statistical dependencies between the variables. Instead, A CPT is useful for speculating about significant events that are about to happen.
Figure 3 shows the propagation algorithm using probabilistic inference with the BN after entering a patient’s status in terms f medical history, diet, and having a retained root. The posterior probabilities of treatment recommendations for systemic disease treatment, surgery or extraction, and prevention were 0.985, 1, and 1, respectively, changing from 0.380, 0.146, and 0.995, respectively, in the initial state shown in
Figure 2.
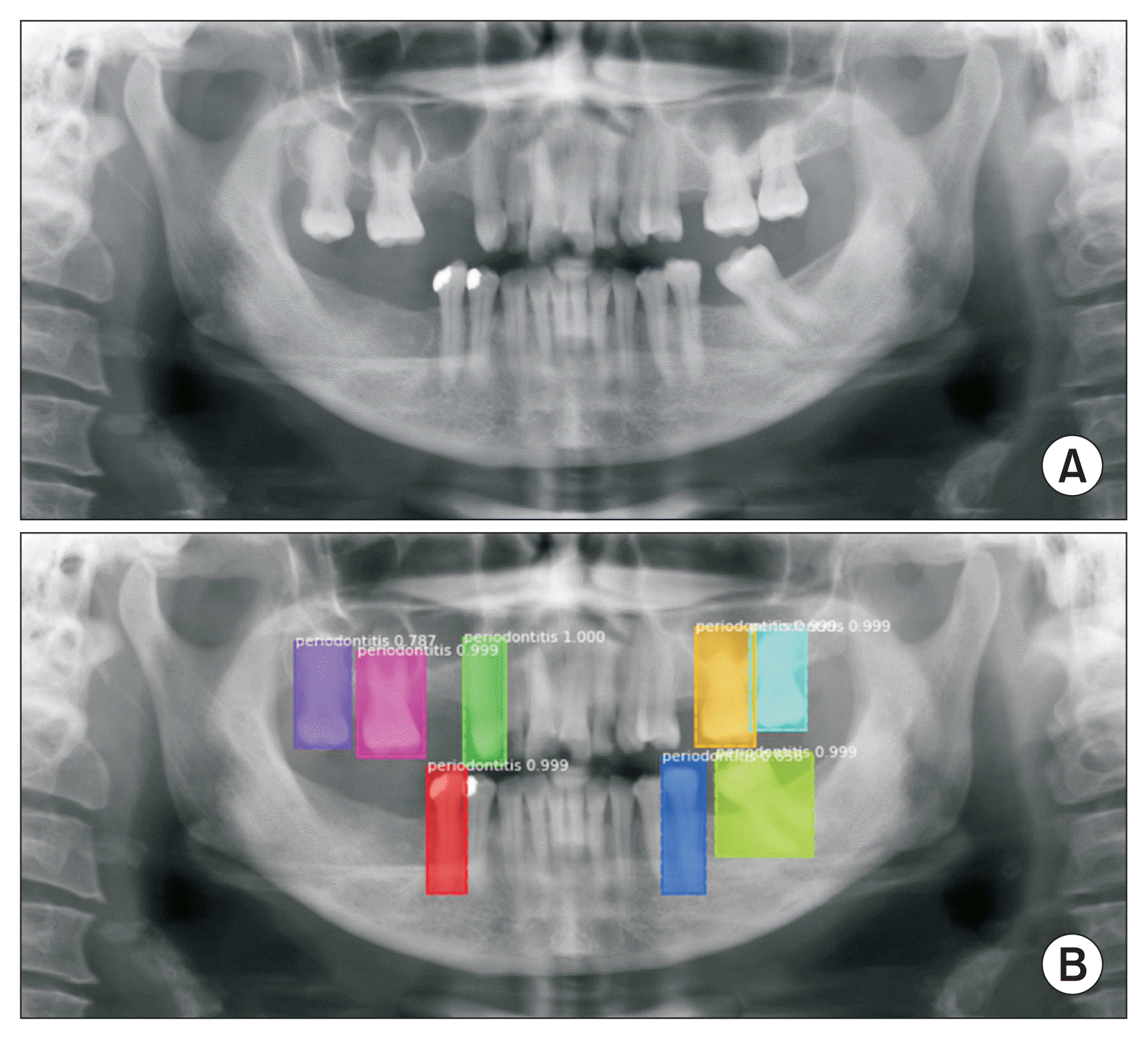 | Figure 1(A) Original panoramic radiograph and (B) the output generated from the faster R-CNN object detection model showing correct predictions of periodontally compromised teeth. R-CNN: regional convolutional neural network. 
|
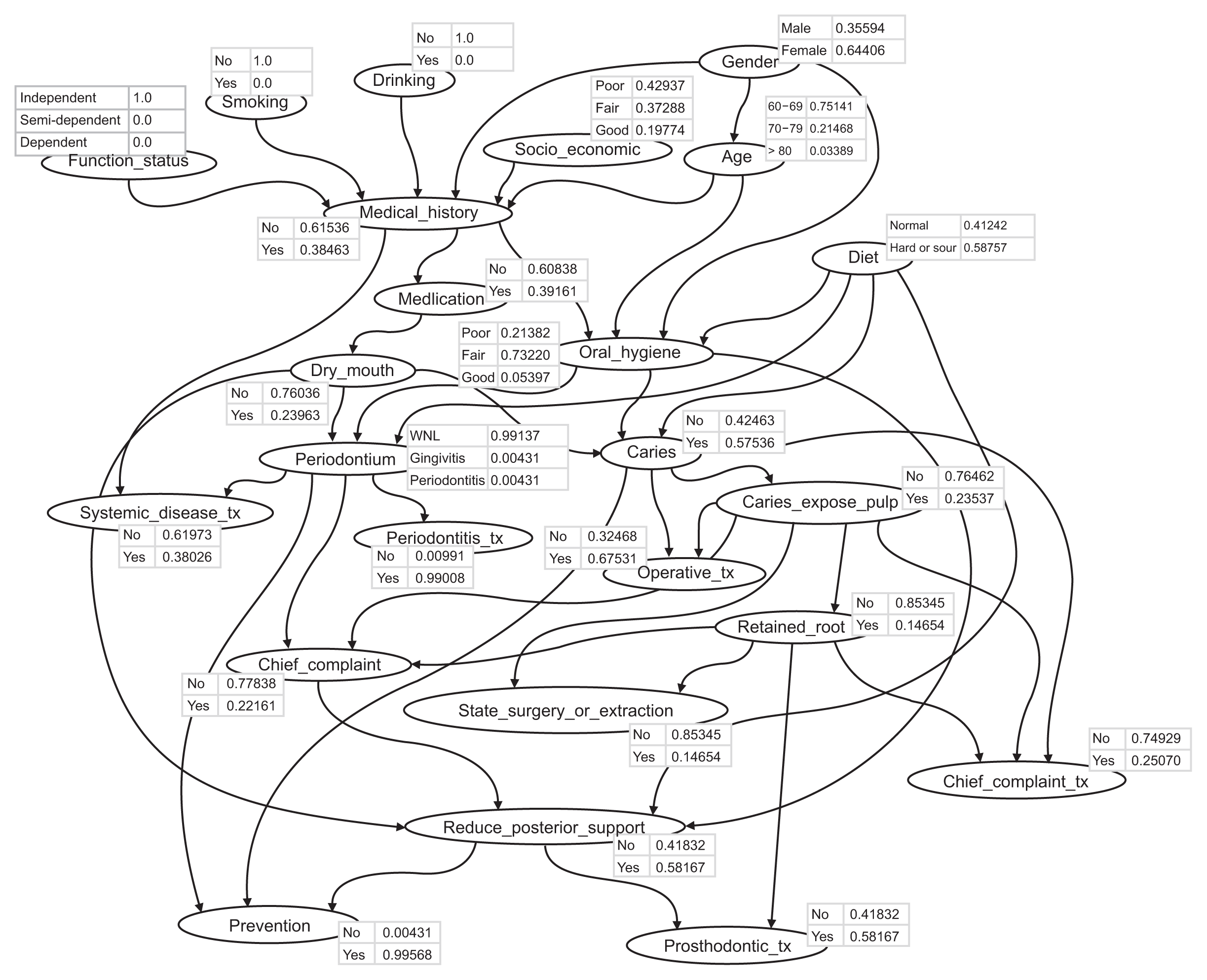 | Figure 2Bayesian network with a conditional probability distribution representing the possible relationships among factors influencing geriatric dental treatment. Each arc indicates a causal relationship. 
|
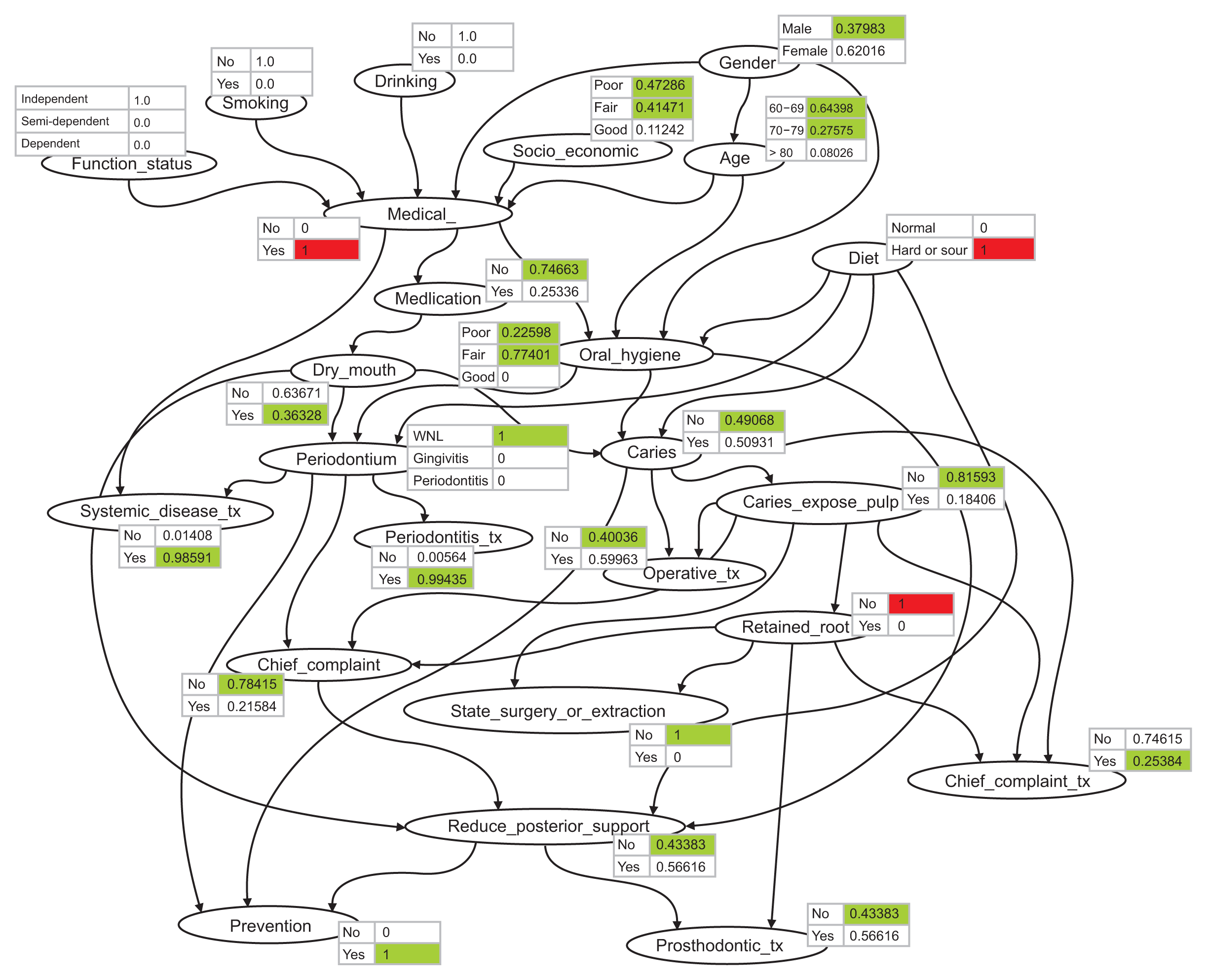 | Figure 3Conditional probability distributions of all nodes after entering the patient status data. 
|
The BN model was highly accurate in recommending therapeutic plans, according to the receiver operating characteristic curve analysis. The AUC was 0.902 in the test dataset (
Figure 4).
Figure 5 shows the software’s graphical user interface when entering data for one patient. The outcome showed that the need for prosthodontic treatment was 1, that for preventive treatment was 0.809, and that for periodontal treatment was 0.805. Data from 20 randomly selected external cases (15 female patients and five male patients) were used to assess the system’s performance. The two experts showed a high level of agreement (kappa = 0.905) for the recommended treatments. The CDSS also showed a high level of agreement with both expert A (kappa = 0.905) and expert B (kappa = 0.827).
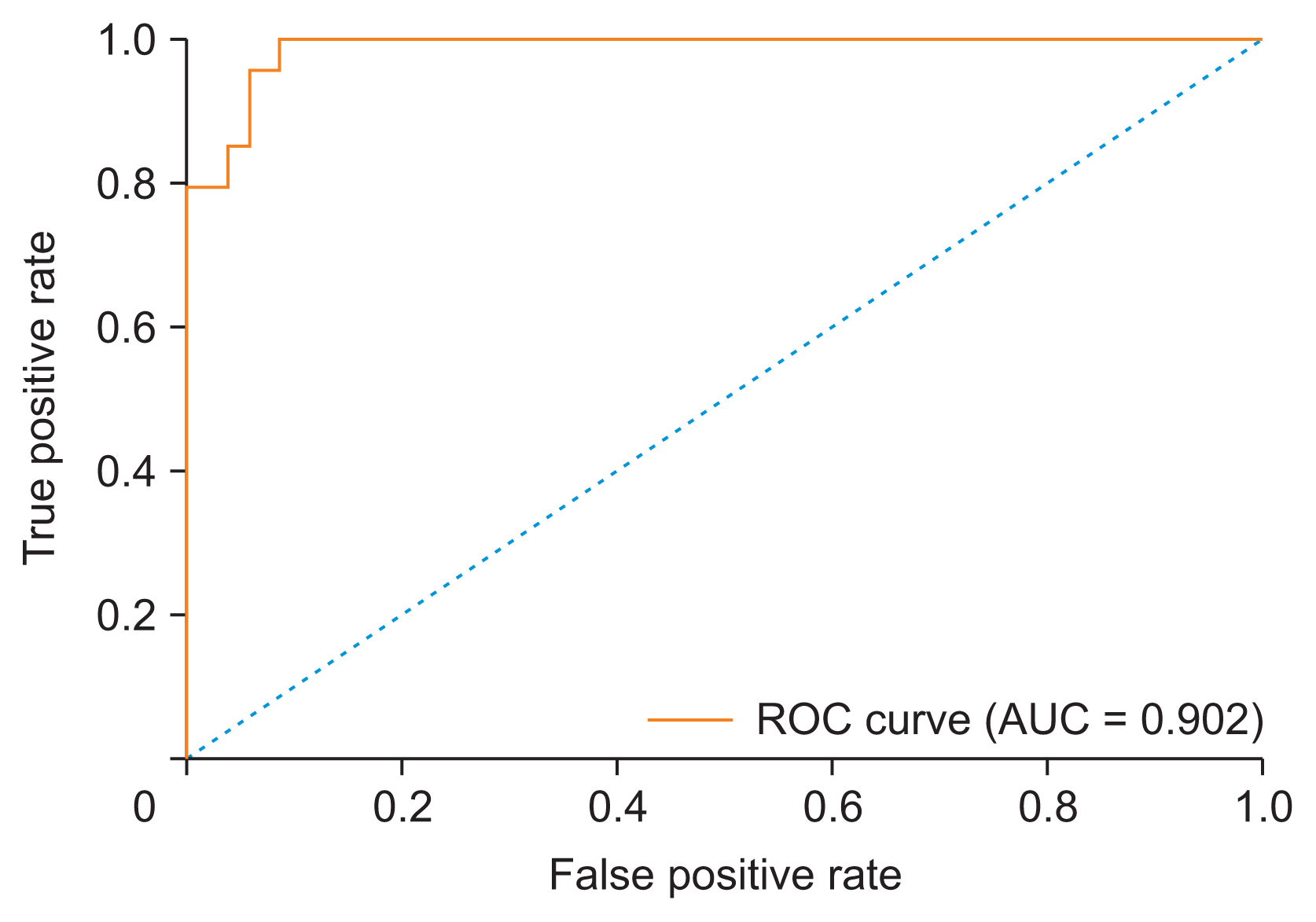 | Figure 4Receiver operating characteristic (ROC) curve of the Bayesian network model, showing an area under the curve (AUC) of 0.902. 
|
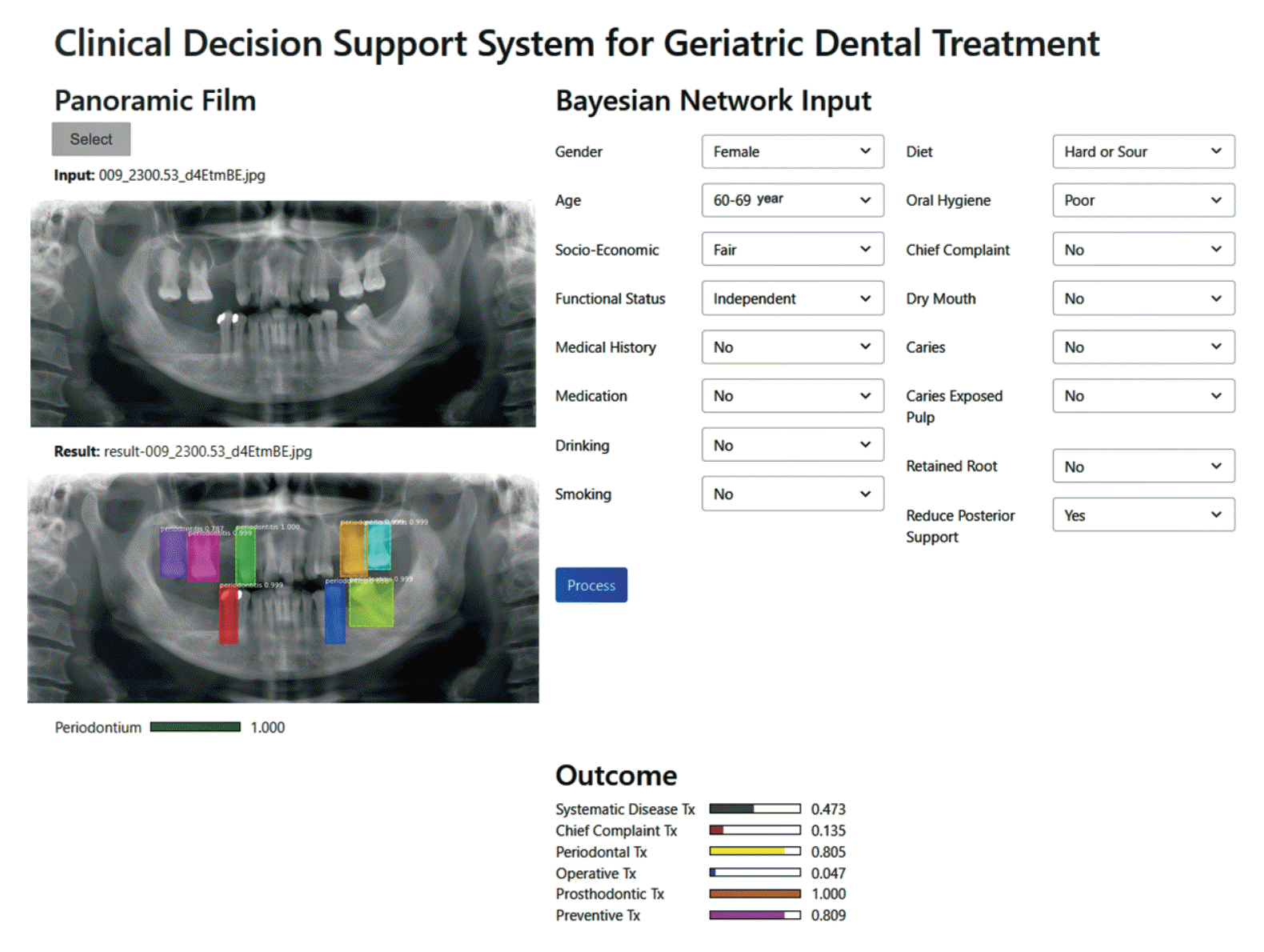 | Figure 5Screenshot of the system user interface showing a recommendation for geriatric dental treatment. 
|
Go to :

IV. Discussion
Clinical decision support is an important part of modern electronic health records that can limit patient care errors and improve evidence-based treatment adherence. Decision support involves finding useful information, making clinical judgments, and providing the best feasible patient care using appropriate technology [
12]. Factors including ease of use, adherence to clinical recommendations, patient-physician cooperation, and integration of electronic health data can all contribute to the impact of a CDSS. People develop many chronic diseases as they age, leading to a high prevalence of dependency. CDSSs develop implementation strategies to assist healthcare professionals in the treatment of geriatric patients in hospitals. CDSS treatments can help elderly patients receive better care in hospitals, with a focus on process-related outcomes [
13]. A review of the use of CDSSs in geriatric care suggested that CDSSs can improve the daily work of healthcare professionals and the clinical outcomes of residents in several disciplines, especially the prevention of pressure ulcers and the improvement of drug prescriptions [
14]. In this study, we developed a CDSS for recommending geriatric dental treatments. The BN-based CDSS consisted of relevant variables based on a comprehensive assessment of dental care. There were three categories of oral health status: the enabling conditions consisted of age, sex, medical history, medication, socioeconomic status, functional status, diet, drinking, and smoking; the faults consisted of oral hygiene, the chief complaint, and dry mouth; and the consequences consisted of periodontal status, caries, caries-exposed pulp, retained root, and reduced posterior support. The proposed CDSS was evaluated by comparing experts’ recommendations for geriatric dentistry treatment to the recommendations made by the system. There was a high degree of consensus between the expert judgment and the results of the proposed system considering the limited training dataset used in this study.
In this study, we incorporated an automatic detection of oral health status using a deep CNN. CNNs have shown unprecedented success in detecting and categorizing abnormalities in medical images [
15]. The algorithm abstractly mimics the brain’s visual function, which processes various data types, such as image dimension, location, and transition [
16]. The CNN architecture process enables the model to accurately classify and detect abnormalities in clinical image data [
17]. Researchers have employed CNN-based object detection algorithms to detect periodontally compromised teeth on digital panoramic radiographs. The detection performance was similar to that of the previous study by Thanathornwong and Suebnukarn [
18]. The faster R-CNN performed satisfactorily in detecting periodontally compromised teeth on digital panoramic radiographs. The automatic detection of periodontally compromised teeth performed by faster R-CNN can save assessment time and reduce diagnostic efforts by enabling automated screening documentation. Researchers have recently reported successfully using deep CNN algorithms to detect caries and alveolar bone lesions on periapical films [
19].
Some limitations in this study need to be addressed. First, the dataset was relatively small since our university’s geriatric dental clinic was only established in 2020. The dataset could be expanded by collecting cases from multiple centers and gathering more image data from an oral screening program via telemedicine. Second, the database contained variables related to the status and interventions for geriatric dental care. More contributing variables related to predisposing factors and clinical data could be incorporated. The clinical data were subject to some degree of uncertainty arising from missing information, noise, and bias [
20]. To overcome data uncertainties, a fuzzy logic system could be integrated with deep learning to solve classification problems [
21]. Third, the detection model only included the periodontal status. Fully automated detection of oral health status (e.g., oral lesions, dental caries, periodontal status, restoration and dental appliances) should be implemented for digital panoramic radiographs and oral photographic images. This automatic detection, combined with clinical data and integrated with electronic health records, is expected to provide decision support for geriatric dental care. The performance of the system could be compared to clinical guidelines in gerontology, as well as treatment recommendations by board-certified dentists. The final system could be evaluated by randomized controlled trials comparing clinicians’ decision-making with the performance of the CDSS for geriatric dental treatment.
This study demonstrated the development and performance of a CDSS for recommending geriatric dental treatment. The BN provided decision support recommendations using comprehensive oral health status. The performance of the system, tested by comparing the results generated by the system with those suggested by experts in geriatric dentistry, was promising. The system and experts in geriatric dentistry strongly agreed on recommended therapeutic plans for elderly patients.
Go to :

Acknowledgments
This work was supported by the research funding from the National Research Council of Thailand.
Go to :

Notes
Go to :

References
6. Patel VL, Shortliffe EH, Stefanelli M, Szolovits P, Berthold MR, Bellazzi R, et al. The coming of age of artificial intelligence in medicine. Artif Intell Med. 2009; 46(1):5–17.
https://doi.org/10.1016/j.artmed.2008.07.017
.
7. Warin K, Limprasert W, Suebnukarn S, Jinaporntham S, Jantana P. Automatic classification and detection of oral cancer in photographic images using deep learning algorithms. J Oral Pathol Med. 2021; 50(9):911–8.
https://doi.org/10.1111/jop.13227
.
10. Ren S, He K, Girshick R, Sun J. Faster R-CNN: towards real-time object detection with region proposal networks. IEEE Trans Pattern Anal Mach Intell. 2017; 39(6):1137–49.
https://doi.org/10.1109/TPAMI.2016.2577031
.
12. Taheri Moghadam S, Sadoughi F, Velayati F, Ehsanzadeh SJ, Poursharif S. The effects of clinical decision support system for prescribing medication on patient outcomes and physician practice performance: a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2021; 21(1):98.
https://doi.org/10.1186/s12911-020-01376-8
.
13. Damoiseaux-Volman BA, van der Velde N, Ruige SG, Romijn JA, Abu-Hanna A, Medlock S. Effect of interventions with a clinical decision support system for hospitalized older patients: systematic review mapping implementation and design factors. JMIR Med Inform. 2021; 9:e28023.
https://doi.org/10.2196/28023
.
14. Abdellatif A, Bouaud J, Lafuente-Lafuente C, Belmin J, Seroussi B. Computerized decision support systems for nursing homes: a scoping review. J Am Med Dir Assoc. 2021; 22:984–94.
https://doi.org/10.1016/j.jamda.2021.01.080
.
17. Panayides AS, Amini A, Filipovic ND, Sharma A, Tsaftaris SA, Young A, et al. AI in medical imaging informatics: current challenges and future directions. IEEE J Biomed Health Inform. 2020; 24(7):1837–57.
https://doi.org/10.1109/JBHI.2020.2991043
.
18. Thanathornwong B, Suebnukarn S. Automatic detection of periodontal compromised teeth in digital panoramic radiographs using faster regional convolutional neural networks. Imaging Sci Dent. 2020; 50(2):169–74.
https://doi.org/10.5624/isd.2020.50.2.169
.
19. Khan HA, Haider MA, Ansari HA, Ishaq H, Kiyani A, Sohail K, et al. Automated feature detection in dental periapical radiographs by using deep learning. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021; 131(6):711–20.
https://doi.org/10.1016/j.oooo.2020.08.024
.
Go to :






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download