Introduction
Methods
1. Study population and treatment
2. Study variables
3. Statistical analysis
Results
1. Clinical characteristics
Table 1.
| Characteristic | DW group (n=26) | HS group (n=38) | p-valuea) |
|---|---|---|---|
| Age (yr) | 59.4±8.0 | 59.8±11.6 | 0.873 |
| Male sex | 25 (96.2) | 31 (81.6) | 0.128 |
| Hemoglobin (g/dL) | 13.2±1.5 | 13.4±1.9 | 0.765 |
| Albumin (g/dL) | 4.1±0.4 | 4.3±0.3 | 0.094 |
| Creatinine (mg/dL) | 0.9±0.3 | 0.8±0.2 | 0.115 |
| Body mass index (kg/m2) | 22.9±3.3 | 23.0±2.7 | 0.851 |
| Location of cancer | 0.955 | ||
| Nasal cavity, nasopharynx | 6 (23.1) | 7 (18.4) | |
| Oral cavity (tonsil, tongue, BOT), oropharynx | 8 (30.8) | 11 (28.9) | |
| Larynx | 10 (38.5) | 17 (44.7) | |
| The others (LN, EAC, unknown primary) | 2 (7.7) | 3 (7.9) | |
| Cumulative dose of cisplatin (mg/m2) | |||
| First chemotherapy | 94.4±12.6 | 91.1±17.1 | 0.393 |
| Second chemotherapy | 182.7±23.2 | 168.4±28.2 | 0.037 |
| Third chemotherapy | 262.1±34.8 | 235.0±34.5 | 0.003 |
2. Changes in hearing thresholds after chemotherapy according to prehydration solution
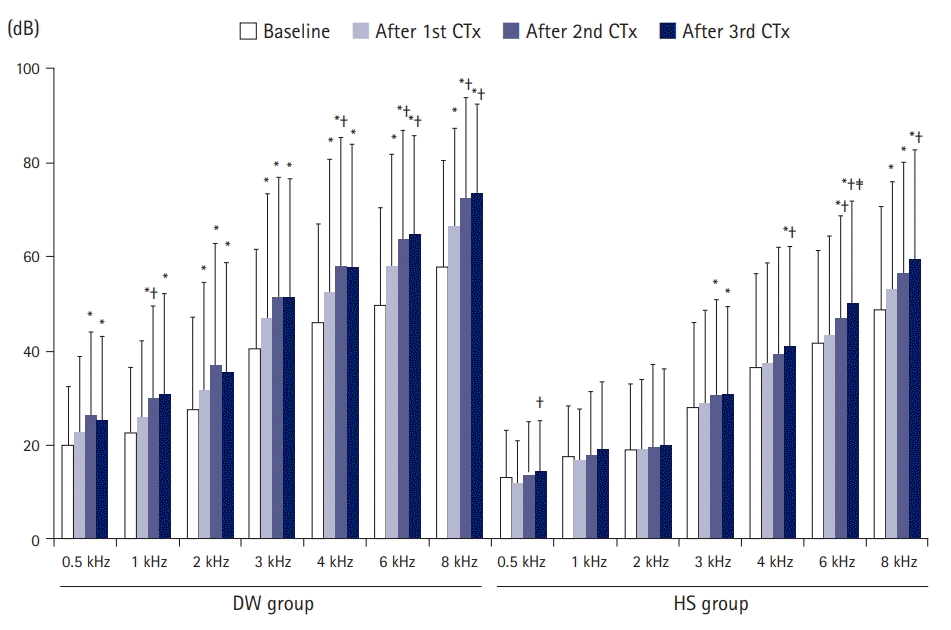 | Fig. 1.Hearing thresholds after CTx according to prehydration solution. The mean 0.5 kHz values at baseline, and after the first CTx, second CTx, and third CTx were 19.8±12.6, 22.5±16.3, 26.2±17.7, and 25.3±17.7 dB for the DW group and 13.0±10.0, 11.8±9.2, 13.5±11.5, and 14.5±10.7 dB for the HS group, respectively. The mean 1 kHz values at baseline, after the first CTx, second CTx, and third CTx were 22.9±13.5, 25.8±16.3, 29.8±19.7, and 30.8±21.4 dB for the DW group and 17.4±10.8, 16.6±11.1, 17.9±13.3, and 19.0±14.3 dB for the HS group. The mean 2 kHz values at baseline, after the first CTx, second CTx, and third CTx were 27.4±19.8, 31.5±23.0, 36.9±25.9, and 35.4±23.2 dB for the DW group and 18.9±14.0, 18.9±15.1, 19.5±17.6, and 20.1±16.0 dB for the HS group. The mean 3 kHz values at baseline, after the first CTx, second CTx, and third CTx were 40.5±21.0, 47.0±26.2, 51.4±25.4, and 51.3±25.3 dB for the DW group and 28.0±18.1, 28.7±19.9, 30.4±20.5, and 30.8±18.5 dB for the HS group. The mean 4 kHz values at baseline, after the first CTx, second CTx, and third CTx were 45.9±21.0, 52.4±28.4, 57.9±27.4, and 57.6±26.3 dB for the DW group and 36.4±19.9, 37.3±21.3, 39.3±22.6, and 40.9±21.2 dB for the HS group. The mean 6 kHz values at baseline, after the first CTx, second CTx, and third CTx were 49.4±21.0, 57.8±23.9, 63.7±23.2, and 64.7±21.0 dB for the DW group and 41.6±19.6, 43.5±20.8, 46.9±21.7, and 50.2±21.6 dB for the HS group. The mean 8 kHz values at baseline, after the first CTx, second CTx, and third CTx were 57.9±22.5, 66.6±20.6, 72.2±21.5, and 73.4±18.8 dB for the DW group and 48.8±21.8, 53.3±22.7, 56.3±23.6, and 59.4±23.3 dB for the HS group. CTx, chemotherapy; DW, dextrose solution; HS, Hartmann solution. *p<0.05 vs. the value at baseline; †p<0.05 vs. the value after the first CTx; ‡p<0.05 vs. the value after the second CTx. |
Table 2.
| Analysis |
After the first chemotherapy from baseline |
After the second chemotherapy from baseline |
After the third chemotherapy from baseline |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DW group | HS group | p-valuea) | DW group | HS group | p-valuea) | DW group | HS group | p-valuea) | |
| Univariate | |||||||||
| 0.5 kHz | 2.7±8.0 | –1.3±4.5 | 0.014 | 6.3±11.1 | 0.5±5.6 | 0.007 | 5.5±13.0 | 1.5±5.8 | 0.102 |
| 1 kHz | 2.9±8.3 | –0.7±4.7 | 0.031 | 6.9±12.1 | 0.5±5.9 | 0.007 | 7.9±14.4 | 1.6±7.6 | 0.028 |
| 2 kHz | 4.1±10.1 | 0.1±5.7 | 0.045 | 9.5±19.3 | 0.6±7.5 | 0.012 | 8.0±17.2 | 1.2±7.9 | 0.036 |
| 3 kHz | 6.5±15.0 | 0.7±7.0 | 0.041 | 11.0±17.8 | 2.4±6.9 | 0.009 | 10.8±18.4 | 2.8±8.5 | 0.023 |
| 4 kHz | 6.5±14.8 | 0.9±5.6 | 0.060 | 12.0±17.2 | 2.9±9.8 | 0.009 | 11.7±15.8 | 4.5±12.7 | 0.048 |
| 6 kHz | 8.4±14.4 | 1.9±8.3 | 0.026 | 14.2±15.9 | 5.3±10.6 | 0.009 | 15.3±15.2 | 8.6±13.4 | 0.069 |
| 8 kHz | 8.8±15.3 | 4.5±9.6 | 0.181 | 14.3±15.6 | 7.6±13.4 | 0.068 | 15.5±15.7 | 10.7±16.8 | 0.251 |
| Multivariate | |||||||||
| 0.5 kHz | 3.2±1.3 | –1.6±1.0 | 0.009 | 6.3±1.8 | 0.5±1.4 | 0.018 | 5.2±2.1 | 1.7±1.7 | 0.221 |
| 1 kHz | 3.4±1.2 | –1.0±1.0 | 0.010 | 6.6±1.8 | 0.7±1.5 | 0.019 | 7.0±2.3 | 2.2±1.9 | 0.129 |
| 2 kHz | 4.5±1.4 | –0.2±1.1 | 0.014 | 10.0±2.8 | 0.2±2.3 | 0.012 | 7.9±2.7 | 1.2±2.1 | 0.070 |
| 3 kHz | 6.8±2.2 | 0.6±1.8 | 0.042 | 10.6±2.7 | 2.7±2.2 | 0.037 | 9.4±2.8 | 3.8±2.3 | 0.152 |
| 4 kHz | 6.2±2.1 | 1.1±1.7 | 0.078 | 11.1±2.7 | 3.5±2.2 | 0.041 | 10.1±2.9 | 5.7 ±2.3 | 0.263 |
| 6 kHz | 8.4±2.1 | 1.9±1.8 | 0.026 | 13.3±2.6 | 6.0±2.1 | 0.038 | 13.4±2.8 | 9.9±2.2 | 0.349 |
| 8 kHz | 9.2±2.3 | 4.2±1.9 | 0.110 | 13.6±2.8 | 8.1±2.2 | 0.143 | 13.7±3.0 | 11.8±2.4 | 0.647 |
Values are presented as mean±standard deviation for univariate analysis and mean±standard error for multivariate analysis. Values were calculated from values obtained after each chemotherapy minus baseline.
DW, dextrose solution; HS, Hartmann solution.
a) The p-values were tested by Student t-test for univariate analysis and analysis of covariance for multivariate analysis. All regimens of chemotherapy were the same excluding doses of cisplatin and radiotherapy. Changes in hearing thresholds after the first, second, and third chemotherapy were compared between the DW and HS groups. The dependent variable was difference in hearing threshold between the baseline and after each cycle of chemotherapy. Covariates were age and baseline hearing thresholds before first chemotherapy, sex, and cumulative dose of cisplatin at the time of audiogram.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download