1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–19.
2. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010; 233:233–55.
3. Matsuno H, Yudoh K, Nakazawa F, Sawai T, Uzuki M, Nishioka K, et al. Antirheumatic effects of humanized anti-Fas monoclonal antibody in human rheumatoid arthritis/SCID mouse chimera. J Rheumatol. 2002; 29:1609–14.
4. Yoo SA, Park JH, Hwang SH, Oh SM, Lee S, Cicatiello V, et al. Placental growth factor-1 and -2 induce hyperplasia and invasiveness of primary rheumatoid synoviocytes. J Immunol. 2015; 194:2513–21.
5. Lefèvre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009; 15:1414–20.
6. Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, et al. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum. 2007; 56:4015–23.
7. Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J Immunol. 2002; 168:5867–75.
8. Marinova-Mutafchieva L, Gabay C, Funa K, Williams RO. Remission of collagen-induced arthritis is associated with high levels of transforming growth factor-beta expression in the joint. Clin Exp Immunol. 2006; 146:287–93.
9. Lee KM, Zhang Z, Achuthan A, Fleetwood AJ, Smith JE, Hamilton JA, et al. IL-23 in arthritic and inflammatory pain development in mice. Arthritis Res Ther. 2020; 22:123.
10. Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005; 12:167–81.
11. Chu CQ, Song Z, Mayton L, Wu B, Wooley PH. IFNgamma deficient C57BL/6 (H-2b) mice develop collagen induced arthritis with predominant usage of T cell receptor Vbeta6 and Vbeta8 in arthritic joints. Ann Rheum Dis. 2003; 62:983–90.
12. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003; 198:1951–7.
13. Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004; 50:650–9.
14. Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice: I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977; 20:841–50.
15. Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001; 1:377–85.
16. Razawy W, Asmawidjaja PS, Mus AM, Salioska N, Davelaar N, Kops N, et al. CD4+ CCR6+ T cells, but not γδ T cells, are important for the IL-23R-dependent progression of antigen-induced inflammatory arthritis in mice. Eur J Immunol. 2020; 50:245–55.
17. Cornelissen F, Mus AM, Asmawidjaja PS, van Hamburg JP, Tocker J, Lubberts E. Interleukin-23 is critical for full-blown expression of a non-autoimmune destructive arthritis and regulates interleukin-17A and RORgammat in gammadelta T cells. Arthritis Res Ther. 2009; 11:R194.
18. Glant TT, Mikecz K, Bartlett RR, Deák F, Thonar EJ, Williams JM, et al. Immunomodulation of proteoglycan-induced progressive polyarthritis by leflunomide. Immunopharmacology. 1992; 23:105–16.
19. Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987; 30:306–18.
20. Kaplan C, Valdez JC, Chandrasekaran R, Eibel H, Mikecz K, Glant TT, et al. Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 2002; 4:54–8.
21. Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Mikecz K, et al. IFN-gamma regulates the requirement for IL-17 in proteoglycan-induced arthritis. J Immunol. 2010; 184:1552–9.
22. Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005; 304:126–36.
23. Nandakumar KS, Bäcklund J, Vestberg M, Holmdahl R. Collagen type II (CII)-specific antibodies induce arthritis in the absence of T or B cells but the arthritis progression is enhanced by CII-reactive T cells. Arthritis Res Ther. 2004; 6:R544–50.
24. Moore AR, Allden S, Bourne T, Denis MC, Kranidioti K, Okoye R, et al. Collagen II antibody-induced arthritis in Tg1278TNFko mice: optimization of a novel model to assess treatments targeting human TNFα in rheumatoid arthritis. J Transl Med. 2014; 12:285.
25. Zhao T, Xie Z, Xi Y, Liu L, Li Z, Qin D. How to model rheumatoid arthritis in animals: from rodents to non-human primates. Front Immunol. 2022; 13:887460.
26. Wang C, Wang W, Jin X, Shen J, Hu W, Jiang T. Puerarin attenuates inflammation and oxidation in mice with collagen antibody-induced arthritis via TLR4/NF-κB signaling. Mol Med Rep. 2016; 14:1365–70.
27. Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991; 10:4025–31.
28. Williams RO, Feldmann M, Maini RN. Cartilage destruction and bone erosion in arthritis: the role of tumour necrosis factor alpha. Ann Rheum Dis. 2000; 59(Suppl I):i75–80.
29. Joe B, Griffiths MM, Remmers EF, Wilder RL. Animal models of rheumatoid arthritis and related inflammation. Curr Rheumatol Rep. 1999; 1:139–48.
30. Kinne RW, Stuhlmüller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007; 9:224.
31. Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, Matsuki T, et al. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Invest. 2004; 114:1603–11.
32. Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000; 191:313–20.
33. Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003; 100:5986–90.
34. Duchosal MA. SCID mice in the study of human autoimmune diseases. Springer Semin Immunopathol. 1992; 14:159–77.
35. Elkon KB, Ashany D. The SCID mouse as a vehicle to study autoimmunity. Br J Rheumatol. 1993; 32:4–12.
36. Schinnerling K, Rosas C, Soto L, Thomas R, Aguillón JC. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front Immunol. 2019; 10:203.
37. Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996; 149:1607–15.
38. You S, Yoo SA, Choi S, Kim JY, Park SJ, Ji JD, et al. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc Natl Acad Sci U S A. 2014; 111:550–5.
39. Dang J, Zhu S, Wang J. A protocol for humanized synovitis mice model. Am J Clin Exp Immunol. 2019; 8:47–52.
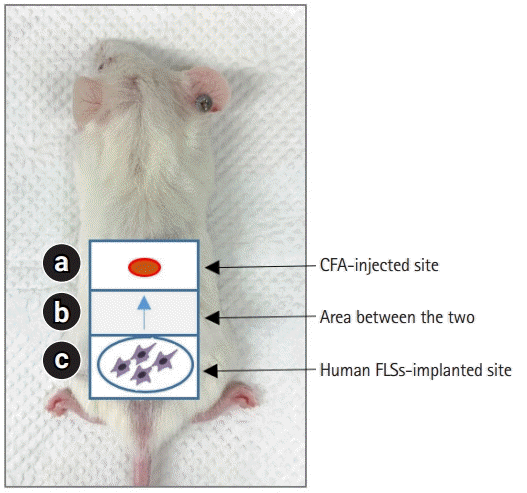
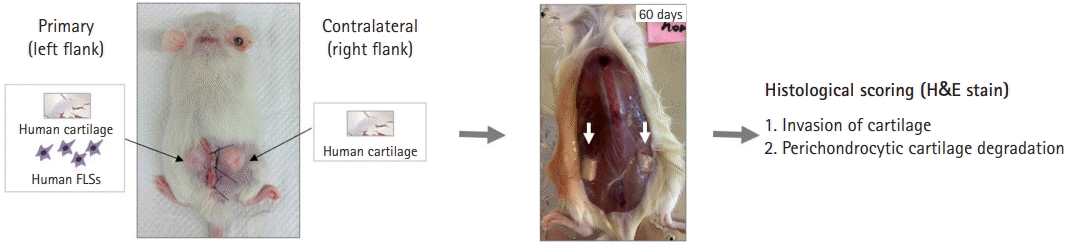




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download