Abstract
Symptomatic extravasation of irrigation fluid is a rare complication of hip arthroscopy. However, depending on the amount of fluid, intra-abdominal hypertension (IAH) may occur and even develop into abdominal compartment syndrome, which can seriously alter hemodynamic circulation. Therefore, it is important for anesthesiologists to promptly recognize the abnormal signs of IAH for early diagnosis and better clinical outcomes. Nevertheless, these signs are difficult to detect because they are usually obscured when the patient is under anesthesia and masked by surgical drapes. We report a case of IAH under general anesthesia during hip arthroscopy to highlight possible symptoms and signs.
Hip arthroscopy has recently become popular because it is a minimally invasive technique with a short recovery time. It is considered a relatively safe procedure because the complication rate is less than 1.5% [1]. Among the complications, the incidence of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) caused by extravasation of irrigation fluid is only 0.16% [2]. Despite their extremely low frequency, these complications can be catastrophic and lead to hemodynamic collapse. In most cases, the physiological signs are difficult to distinguish from those of other conditions, although a clinical diagnosis must be made. We report a rare case of IAH that occurred during hip arthroscopy under general anesthesia.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital (IRB No: 2022-06-004). Patient consent was obtained for the publication of this case report.
A 56-year-old woman (height, 155 cm; weight, 45 kg) with chronic osteoarthritis was scheduled to undergo arthroscopy of the right hip joint and labral repair for excision of a right ganglion cyst under general anesthesia. The patient was otherwise healthy and had no other disease. Additionally, laboratory test results were normal. Standard monitoring was performed in the operating room, including noninvasive blood pressure (BP), electrocardiography, bispectral index, body temperature, and pulse oximetry monitoring. General anesthesia was induced and maintained with lidocaine, propofol, remifentanil, rocuronium, and desflurane. Arthroscopy was performed with the patient in the supine position. Subsequently, anterior, anterolateral, and posterior ports were inserted. The irrigation fluid was prepared by mixing 3 L of 0.9% sodium chloride solution with 2 mg of epinephrine. A total of 25 L of irrigation fluid was applied, and the patient's vital signs remained stable throughout the surgery.
However, 3 hours after commencing the surgery, when suturing was almost complete, her systolic BP suddenly decreased from 130 to 80 mmHg, with an increase in peak inspiratory pressure (PIP) from 15 to 24 mmHg. We initially suspected bronchial spasm or embolism. However, auscultation was unremarkable, and the end-tidal CO2 curves did not show any significant changes, including signs of spontaneous breathing recovery. Her BP returned to baseline after phenylephrine administration. However, her increased PIP level did not normalize even after endotracheal tube suction, albuterol application, and lung recruitment. Additionally, a sudden decrease in body temperature, which had been maintained at approximately 36.5°C during the surgery, was detected. However, we initially overlooked this sign because the decrease was not large, from 36.7°C to 35.0°C, and body temperature tends to drop gradually during arthroscopy. Moreover, we assumed that the position of the esophageal temperature probe tip might have changed during surgery. Despite the abrupt changes in PIP and body temperature, we decided to awaken the patient because all other vital signs seemed stable. When the surgical drapes were removed, abdominal distention was observed, indicating IAH. Arterial blood gas was analyzed to differentiate between hemorrhage and irrigation fluid. The hematocrit (34.8%) and electrolyte (sodium, 135 mmol/L; potassium, 3.5 mmol/L) values were unremarkable. However, metabolic acidosis was confirmed (pH, 7.295; HCO3–, 16.8; base excess, –8.5). Based on these findings, an accumulation of irrigation fluid was highly suspected to be causing the abdominal distention rather than bleeding. After discussion with the orthopedic surgeons, we decided to consult general surgeons postoperatively because the latter were not immediately available. Subsequently, sugammadex was administered to reverse muscle relaxation. Approximately 5 to 8 minutes later, extubation was performed upon complete recovery of spontaneous breathing, and the patient seemed to recover from sedation. No remarkable events occurred during the emergence period.
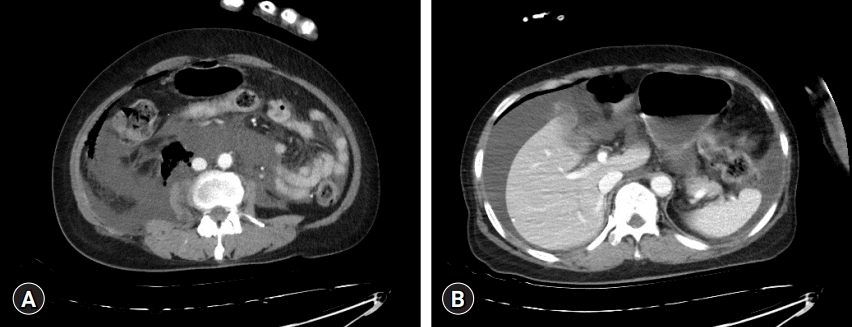
However, as the patient regained consciousness, although her vital signs were stable and she could breathe without discomfort, she experienced abdominal pain. Ketorolac was administered for pain control, and intravenous fluid administration was restricted in the postoperative care unit. The patient was transferred for confirmation using abdominal computed tomography (CT), which revealed a large amount of fluid in the abdominal cavity, particularly around the right perihepatic space (Fig. 1). According to the general surgeon, emergent surgical decompression was not indicated in this case. Percutaneous catheter drainage was performed in the right perihepatic space. After approximately 1.5 L of fluid was drained from the catheter, the patient's symptoms improved. On the second postoperative day, a follow-up CT scan showed no residual fluid. She was discharged 6 days later without any further symptoms or complications. Patient consent was obtained for publication of this case report.
Although hip arthroscopy is considered to be a relatively safe procedure, more complications have been reported as its popularity has increased. Haskins et al. [3] recently reported extravasation of irrigation fluid in 16% of patients who underwent hip arthroscopy. Among them, the incidence of symptomatic intraperitoneal accumulation of irrigation fluid was only 0.16% [2]. In most cases, this can result in increased postoperative pain [3]. However, careful attention must be paid to this complication because, depending on the amount of fluid accumulated, it can be fatal, and even a case of cardiac arrest has been reported [4].
IAH is defined as a sustained increase in intra-abdominal pressure of ≥12 mmHg; if the pressure exceeds 20 mmHg, ACS can develop with organ dysfunction [5]. However, the mechanism by which irrigation fluid accumulates in the intraperitoneal space remains unclear. Once the tendon sheath of the iliopsoas muscle is opened, irrigation fluid can flow into the retroperitoneal space. Verma and Sekiya [6] hypothesized that occasional congenital communication between the retroperitoneum and peritoneal space may be present, which functions as an entrance gate. In addition, there is always a possible risk of the anchor perforating the anteromedial cortex of the acetabular dome and iliopsoas muscle during hip arthroscopy, allowing irrigation fluid to flow into the psoas tunnel [7,8]. High perfusion pressure is also a known risk factor for IAH [8]. The amount or pressure of irrigation fluids should be checked, and if baseline values are exceeded, hemodynamic variations should be carefully monitored. According to Papavasiliou and Bardakos [9], an inflow fluid pressure of 40 to 50 mmHg for hip arthroscopy minimizes the risk of fluid extravasation. Every other condition related to diminished abdominal wall compliance and increased intra-abdominal contents, such as a history of abdominal surgery and obesity, can be considered a tentative risk [10]. Therefore, anesthesiologists should be vigilant in checking if excessive irrigation fluid pressure is used, especially when the patient has risk factors for IAH.
Similar to other compartment syndromes of the extremities, ACS is also a clinical diagnosis [11]. However, many institutions, including our institution, do not perform real-time abdominal pressure monitoring. Therefore, an awareness of the physiological signs of IAH is important. Abdominal extension and discomfort are the most common symptoms. However, these are usually hidden during surgery, as the patient is under anesthesia and the surgical drapes cover the abdominal area. Increased PIP and decreased oxygen saturation, BP, urine output, and body temperature have been commonly reported in similar cases [3,6]. As the pressure in the abdominal cavity increases, the diaphragm may be pushed upward, while the renal parenchyma and vessels are compressed. Additionally, as abdominal vessels are compressed, stroke volume can be affected by reduced venous return and increased peripheral vascular resistance. In our case, although saturation and urine output were unremarkable, sudden changes in PIP and BP were detected. However, these findings were not sufficiently specific to immediately suspect IAH. Thromboembolism and other hemorrhagic or respiratory events also share these features. Therefore, the differential diagnoses included hypovolemia, respiratory spasm, and embolic events. The accuracy could have improved if bronchoscopy had been applied to evaluate other respiratory conditions. However, the procedure was not available during this period at our center. With the help of end-tidal CO2 monitoring and arterial blood gas analysis, we were able to exclude other differential diagnoses.
However, it was difficult to suspect IAH until the surgical cover was removed and abdominal distention was detected, as in many other reported cases [1,12]. Therefore, anesthesiologists must be aware of all IAH features. Sudden hypothermia should be considered as an important diagnostic sign. Although some degree of hypothermia is commonly observed during hip arthroscopy, there is only a slight difference. Typically, the patient’s temperature during surgery tends to decrease gradually and linearly [13,14]. However, in the case of IAH, a sudden and dramatic decrease in the temperature has been observed. Several studies have highlighted the importance of this sign. Bartlett et al. [4] and Sharma et al. [15] reported a sudden decrease in temperature to 33.3°C. However, our patient showed a decrease of 1.7°C, which was not large. However, we believe that the magnitude of decrease can differ depending on the severity of extravasation; therefore, attention should be paid to a sudden change within several minutes. Early observation of this change can be an important additional clue to other findings and differential diagnosis.
Several management strategies have been suggested to reduce intra-abdominal pressure. For anesthesiologists, improved abdominal wall compliance can be expected by appropriate sedation with analgesia and neuromuscular blockade [10]. In our case, we maintained lower intra-abdominal pressure by maintaining sedation and muscle relaxation until the completion of drainage. However, the consulting general surgeon was not available immediately; therefore, CT was performed to determine further treatment. Surgical decompression for IAH should be considered when intra-abdominal pressure is >20 mmHg and new organ dysfunction is present [10]. CT is an accurate method to confirm the possibility of damage to other organs. However, we assume that if vital signs are stable, such as in our case, perioperative ultrasonography can be a useful tool for the early and reliable diagnosis of fluid accumulation. With early application of ultrasonography and drainage, the patient would have experienced less pain, even if she had needed further CT evaluation.
In summary, hip arthroscopy has progressed over the decades and has recently become a more common procedure. Therefore, anesthesiologists must be aware of the complications that may occur. Despite its rare incidence, when physiological alterations that are highly suggestive of IAH occur, prompt inspection should be performed to prevent ACS from occurring with devastating results. Early diagnosis of IAH is essential to prevent this event; however, its detection remains challenging. Herein, we report a case of IAH during hip arthroscopy to highlight the physiological signs that contributed to earlier diagnosis and improved clinical outcomes.
References
1. Fowler J, Owens BD. Abdominal compartment syndrome after hip arthroscopy. Arthroscopy. 2010; 26:128–30.
2. Ciemniewska-Gorzela K, Piontek T, Szulc A. Abdominal compartment syndrome: the prevention and treatment of possible lethal complications following hip arthroscopy: a case report. J Med Case Rep. 2014; 8:368.
3. Haskins SC, Desai NA, Fields KG, Nejim JA, Cheng S, Coleman SH, et al. Diagnosis of intraabdominal fluid extravasation after hip arthroscopy with point-of-care ultrasonography can identify patients at an increased risk for postoperative pain. Anesth Analg. 2017; 124:791–9.
4. Bartlett CS, DiFelice GS, Buly RL, Quinn TJ, Green DS, Helfet DL. Cardiac arrest as a result of intraabdominal extravasation of fluid during arthroscopic removal of a loose body from the hip joint of a patient with an acetabular fracture. J Orthop Trauma. 1998; 12:294–9.
5. Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006; 32:1722–32.
6. Verma M, Sekiya JK. Intrathoracic fluid extravasation after hip arthroscopy. Arthroscopy. 2010; 26(9 Suppl):S90–4.
7. Yalamanchili DR, Shively S, Banffy MB, Taliwal N, Clark E, Hunter G, et al. Calculating intraoperative fluid deficit to prevent abdominal compartment syndrome in hip arthroscopy. Arthrosc Tech. 2021; 11:e89–93.
8. Kocher MS, Frank JS, Nasreddine AY, Safran MR, Philippon MJ, Sekiya JK, et al. Intra-abdominal fluid extravasation during hip arthroscopy: a survey of the MAHORN group. Arthroscopy. 2012; 28:1654–60.
9. Papavasiliou AV, Bardakos NV. Complications of arthroscopic surgery of the hip. Bone Joint Res. 2012; 1:131–44.
10. Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013; 39:1190–206.
11. Shakuo T, Bito K, Yasuda S, Asagi C. Abdominal compartment syndrome during hip arthroscopy for an acetabular fracture: a case report. JA Clin Rep. 2017; 3:24.
12. Schwenter A, Schuepfer G, Beck M, Mauch J. Abdominal compartment syndrome after hip arthroscopy. Anaesthesist. 2021; 70:398–400.
13. Díaz M, Becker DE. Thermoregulation: physiological and clinical considerations during sedation and general anesthesia. Anesth Prog. 2010; 57:25–32.
14. Bindu B, Bindra A, Rath G. Temperature management under general anesthesia: compulsion or option. J Anaesthesiol Clin Pharmacol. 2017; 33:306–16.
15. Sharma A, Sachdev H, Gomillion M. Abdominal compartment syndrome during hip arthroscopy. Anaesthesia. 2009; 64:567–9.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download