Abstract
Background
Methods
Results
Conclusion
References
Fig. 1.
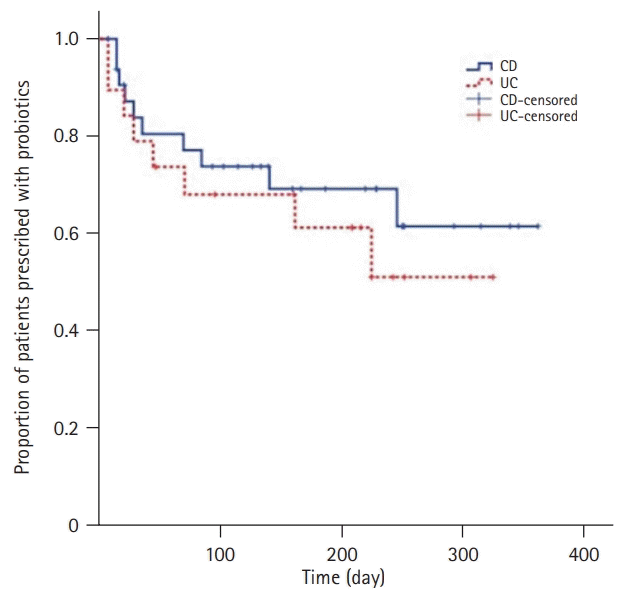
Table 1.
| Characteristic | Probiotics group | Control group | p-value |
|---|---|---|---|
| No. of patients | 52 | 165 | |
| Age (yr) | 34.5±15.7 | 38.5±13.1 | 0.069 |
| Sex | |||
| Male | 33 (63.5) | 108 (65.5) | 0.793 |
| Female | 19 (36.5) | 57 (34.5) | |
| Disease duration (yr) | 22.3±40.3 | 8.7±13.5 | 0.020 |
| Body mass index (kg/m2) | 22.2±3.9 | 22.6±3.6 | 0.492 |
| Duration of probiotics prescription (day) | 180.2±127.3 | ||
| Type of probiotics | |||
| Lacidofil | 21 (40.4) | ||
| Medilac | 15 (28.8) | ||
| Bioflor | 13 (25.0) | ||
| Ramnos | 3 (5.8) | ||
| Type of IBD | |||
| Crohn disease | 33 (63.5) | 54 (32.7) | <0.001 |
| Ulcerative colitis | 19 (36.5) | 111 (67.3) | |
| Physician global assessment | |||
| Remission or mild | 46 (88.5) | 153 (92.7) | 0.331 |
| Moderate or severe | 6 (11.5) | 12 (7.3) | |
| Stool frequency (/day) | 2.93±2.15 | 2.13±1.48 | 0.014 |
| Stool consistency | |||
| Normal | 31 (59.6) | 117 (70.9) | 0.127 |
| Loose stool or diarrhea | 21 (40.4) | 48 (29.1) | |
| Abdominal pain | |||
| Absence or mild | 38 (73.1) | 157 (95.2) | <0.001 |
| Moderate or severe | 14 (26.9) | 8 (4.8) | |
| Hematocheziaa) | |||
| Absence | 43 (84.3) | 127 (77.0) | 0.263 |
| Presence | 8 (15.7) | 38 (23.0) | |
| History of IBD-related surgeries | |||
| Presence | 18 (34.6) | 25 (15.2) | 0.002 |
| Absence | 34 (65.4) | 140 (84.8) | |
| Drug complianceb) | |||
| Good | 45 (90.0) | 146 (89.0) | 0.845 |
| Poor | 5 (10.0) | 18 (11.0) | |
| C-reactive protein (mg/L) | 10.5±17.31 | 4.17±12.3 | 0.027 |
| ESR (mm/hr) | 18.7±22.4 | 15.8±16.7 | 0.451 |
| Hemoglobin (g/dL) | 13.7±2.3 | 13.8±1.6 | 0.788 |
| Total protein (g/dL) | 7.2±0.6 | 11.6±55.5 | 0.599 |
Values are presented as number only, mean±standard deviation, or number (%).
IBD, inflammatory bowel disease; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.
Table 2.
| Characteristic | Probiotics group (n=33) | Control group (n=54) | p-valuea) | OR (95% CI) | p-valueb) |
|---|---|---|---|---|---|
| Age (yr) | 31.5±12.6 | 33.5±11.2 | 0.461 | ||
| Sex | |||||
| Male | 24 (72.7) | 42 (77.8) | 0.593 | ||
| Female | 9 (27.3) | 12 (22.2) | |||
| Disease duration (yr) | 22.6±40.1 | 9.1±15.8 | 0.074 | ||
| Body mass index (kg/m2) | 22.0±4.1 | 21.8±3.1 | 0.804 | ||
| Disease location | |||||
| Small bowel | 16 (48.5) | 14 (25.9) | 0.034 | ||
| Colon | 1 (3.0) | 2 (3.7) | |||
| Small bowel and colon | 16 (48.5) | 38 (70.4) | |||
| Disease behavior | |||||
| Inflammatory | 18 (54.5) | 33 (61.1) | 0.810 | ||
| Fistulating | 11 (33.3) | 13 (24.1) | |||
| Stenosis | 4 (12.1) | 8 (14.8) | |||
| Duration of probiotics prescription (day) | 196.1±134.5 | ||||
| Types of probiotics | |||||
| Lacidofil | 12 (36.4) | ||||
| Medilac | 8 (24.2) | ||||
| Bioflor | 3 (9.1) | ||||
| Ramnos | 10 (30.3) | ||||
| Fistula | |||||
| Presence | 18 (54.5) | 29 (53.7) | 0.939 | ||
| Absence | 15 (45.5) | 25 (46.3) | |||
| Physician global assessment | |||||
| Remission or mild | 29 (87.9) | 52 (96.3) | 0.133 | ||
| Moderate or severe | 4 (12.1) | 2 (3.7) | |||
| CDAI | |||||
| <150 | 29 (87.9) | 52 (96.3) | 0.133 | ||
| Mildc) | 4 (12.1) | 2 (3.7) | |||
| Stool frequency (/day) | 2.8±1.8 | 1.7±1.0 | 0.002 | 2.458 (1.381–4.374) | 0.002 |
| Stool consistency | |||||
| Normal | 21 (63.6) | 39 (72.2) | 0.401 | ||
| Loose stool or diarrhea | 12 (36.4) | 15 (27.8) | |||
| Abdominal pain | |||||
| Absence or mild | 24 (72.7) | 52 (96.3) | 0.001 | 1 | |
| Moderate or severe | 9 (27.3) | 2 (3.7) | 81.846 (5.707–173.788) | 0.001 | |
| History of IBD-related surgeries | |||||
| Presence | 18 (54.5) | 19 (35.2) | 0.076 | 4.588 (1.233–7.068) | 0.023 |
| Absence | 15 (45.5) | 35 (64.8) | 1 | ||
| Drug complianced) | |||||
| Good | 28 (87.5) | 48 (88.9) | 0.846 | ||
| Poor | 4 (12.5) | 6 (11.1) | |||
| Prior biologics use | |||||
| Presence | 12 (36.4) | 20 (37.0) | 0.950 | 0.158 (0.030–0.825) | 0.029 |
| Absence | 21 (63.6) | 34 (63.0) | 1 | ||
| Prior oral 5-ASA use | |||||
| Presence | 33 (100) | 50 (92.6) | 0.109 | ||
| Absence | 0 (0) | 4 (7.4) | |||
| Prior topical 5-ASA use | |||||
| Presence | 3 (9.1) | 2 (3.7) | 0.295 | ||
| Absence | 30 (90.9) | 52 (96.3) | |||
| Prior thiopurine use | |||||
| Presence | 21 (63.6) | 47 (87.0) | 0.010 | 0.206 (0.048–0.891) | 0.035 |
| Absence | 12 (36.4) | 7 (13.0) | 1 | ||
| Prior corticosteroid use | |||||
| Presence | 15 (45.5) | 31 (57.4) | 0.278 | ||
| Absence | 18 (54.5) | 23 (42.6) | |||
| Prior methotrexate use | |||||
| Presence | 5 (15.2) | 1 (1.9) | 0.018 | 31.702 (2.016–498.540) | 0.014 |
| Absence | 28 (84.8) | 53 (98.1) | 1 | ||
| Prior iron use | |||||
| Presence | 14 (42.4) | 11 (20.4) | 0.027 | 15.054 (2.963–76.475) | 0.001 |
| Absence | 19 (57.6) | 43 (79.6) | 1 | ||
| Prior psychotropic drug use | |||||
| Presence | 3 (9.1) | 1 (1.9) | 0.118 | ||
| Absence | 30 (90.9) | 53 (98.1) | |||
| C-reactive protein (mg/L) | 11.9±18.3 | 7.7±19.4 | 0.319 | ||
| ESR (mm/hr) | 17.3±22.7 | 18.1±21.1 | 0.870 | ||
| Hemoglobin (g/dL) | 13.7±2.5 | 13.7±1.8 | 0.938 | ||
| Total protein (g/dL) | 7.0±0.6 | 7.1±0.5 | 0.683 |
Values are presented as mean±standard deviation or number (%) unless otherwise specified.
OR, odds ratio; CI, confidence interval; CDAI, Crohn disease activity index; IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.
Table 3.
| Characteristic | Probiotics group (n=19) | Control group (n=111) | p-valuea) | OR (95% CI) | p-valueb) |
|---|---|---|---|---|---|
| Age (yr) | 39.6±19.2 | 40.9±13.3 | 0.778 | ||
| Sex | |||||
| Male | 9 (47.4) | 66 (59.5) | 0.324 | ||
| Female | 10 (52.6) | 45 (40.5) | |||
| Disease duration (yr) | 21.8±41.7 | 8.5±12.4 | 0.183 | ||
| Body mass index (kg/m2) | 22.6±3.6 | 23.0±3.7 | 0.685 | ||
| Disease location | |||||
| Proctosigmoiditis | 11(57.9) | 51 (45.9) | 0.328 | ||
| Left-sided | 1 (5.3) | 16 (14.4) | |||
| Extensive | 7 (36.8) | 44 (39.6) | |||
| Duration of probiotics prescription (day) | 152.6±111.7 | ||||
| Types of probiotics | |||||
| Lacidofil | 9 (47.4) | ||||
| Medilac | 7 (36.8) | ||||
| Bioflor | 3 (15.8) | ||||
| Ramnos | 0 (0) | ||||
| Physician global assessment | |||||
| Remission or mild | 17 (89.5) | 101 (91.0) | 0.688 | 1 | |
| Moderate or severe | 2 (10.5) | 10 (9.0) | 0.006 (0.000–0.291) | 0.010 | |
| Partial Mayo score | |||||
| Remission | 8 (42.1) | 75 (67.6) | 0.156 | ||
| Mild | 9 (47.4) | 26 (23.4) | |||
| Moderate | 1 (5.3) | 6 (5.4) | |||
| Severe | 1 (5.3) | 4 (3.6) | |||
| Stool frequency (/day) | 3.2±2.7 | 2.3±1.6 | 0.180 | 2.069 (1.256–3.409) | 0.004 |
| Stool consistency | |||||
| Normal | 10 (52.6) | 78 (70.3) | 0.129 | 1 | |
| Loose stool or diarrhea | 9 (47.4) | 33 (29.7) | 0.088 (0.007–1.137) | 0.063 | |
| Abdominal pain | |||||
| Absence or mild | 14 (73.7) | 105 (94.6) | 0.002 | 1 | |
| Moderate or severe | 5 (26.3) | 6 (5.4) | 44.705 (3.683–542.598) | 0.003 | |
| Hematocheziac) | |||||
| Absence | 11 (61.1) | 83 (74.8) | 0.227 | 1 | |
| Presence | 7 (38.9) | 28 (25.2) | 10.479 (1.042–105.376) | 0.046 | |
| History of IBD-related surgeries | |||||
| Presence | 0 (0) | 6 (5.4) | 0.299 | ||
| Absence | 19 (100) | 105 (94.6) | |||
| Drug complianced) | |||||
| Good | 17 (94.4) | 98 (89.1) | 0.486 | ||
| Poor | 1 (5.6) | 12 (10.9) | |||
| Prior biologics use | |||||
| Presence | 3 (15.8) | 18 (16.2) | 0.963 | ||
| Absence | 16 (84.2) | 93 (83.8) | |||
| Prior oral 5-ASA use | |||||
| Presence | 18 (94.7) | 106 (95.5) | 0.884 | ||
| Absence | 1 (5.3) | 5 (4.5) | |||
| Prior topical 5-ASA use | |||||
| Presence | 15 (78.9) | 90 (81.1) | 0.827 | ||
| Absence | 4 (21.1) | 21 (18.9) | |||
| Prior thiopurine use | |||||
| Presence | 3 (15.8) | 33 (29.7) | 0.210 | 0.214 (0.035–1.301) | 0.094 |
| Absence | 16 (84.2) | 78 (70.3) | 1 | ||
| Prior corticosteroid use | |||||
| Presence | 11 (57.9) | 51 (45.9) | 0.335 | ||
| Absence | 8 (42.1) | 60 (54.1) | |||
| Prior iron use | |||||
| Presence | 1 (5.3) | 9 (8.1) | 0.667 | ||
| Absence | 18 (94.7) | 102 (91.9) | |||
| Prior psychotropic drug use | |||||
| Presence | 2 (10.5) | 5 (4.5) | 0.283 | ||
| Absence | 17 (89.5) | 106 (95.5) | |||
| C-reactive protein (mg/L) | 6.2±13.8 | 2.2±4.6 | 0.367 | ||
| ESR (mm/hr) | 22.8±22.0 | 14.5±13.6 | 0.088 | ||
| Hemoglobin (g/dL) | 13.8±1.9 | 13.9±1.5 | 0.810 | ||
| Total protein (g/dL) | 7.5±0.7 | 13.9±68.0 | 0.756 |
Values are presented as mean±standard deviation or number (%) unless otherwise specified.
OR, odds ratio; CI, confidence interval; IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download