Abstract
Caloric restriction is a popular approach to treat obesity and its associated chronic illnesses but is difficult to maintain for a long time. Intermittent fasting is an alternative and easily applicable dietary intervention for caloric restriction. Moreover, intermittent fasting has beneficial effects equivalent to those of caloric restriction in terms of body weight control, improvements in glucose homeostasis and lipid profiles, and anti-inflammatory effects. In this review, the beneficial effects of intermittent fasting are discussed.
Go to : 
Obesity poses a public health risk worldwide because of its association with metabolic dysregulation such as insulin resistance, hypertension, dyslipidemia, and atherosclerosis [1,2]. Caloric restriction (CR) without malnutrition is the cornerstone for the treatment of obesity and its associated metabolic risk factors. It is well known that prolonged CR reduces body weight and extends life expectancy [3,4]. Moreover, CR in obese subjects improves cardiovascular risk factors, insulin sensitivity, and mitochondrial function [5-10]. However, long-term daily CR is difficult to adhere to in practice [11].
Recently, many studies have reported that intermittent CR (intermittent fasting, IF) may improve dietary adherence; thus, IF has emerged as an alternative intervention for prolonged CR, with similar benefits in body weight reduction and chronic illness control [12-19]. IF originated from religious traditions, such as Ramadan fasting [20]. Muslims fast during the daytime (approximately 15 hours between sunrise and sunset) for a month during the Ramadan period every year. Ramadan fasting has been reported to improve human health [21]. IF involves reduced or no caloric intake in an intermittent pattern, such as short periods of very restricted caloric intake or fasting interspersed with normal caloric intake. Thus, dieter intake is 0 to 500 kcal/day on fasting days. The fasting time varies from several hours per day to a complete day. The most studied IF interventions include 2 days of CR or fasting per week (5:2 diet) and alternate-day fasting (ADF) [22]. One of the most popular variants of IF is time-restricted feeding, in which energy intake is limited to 12 to 16 hours each day and normal caloric intake during the other hours. In this review, we evaluate the results mainly from ADF and 5:2 diet trials.
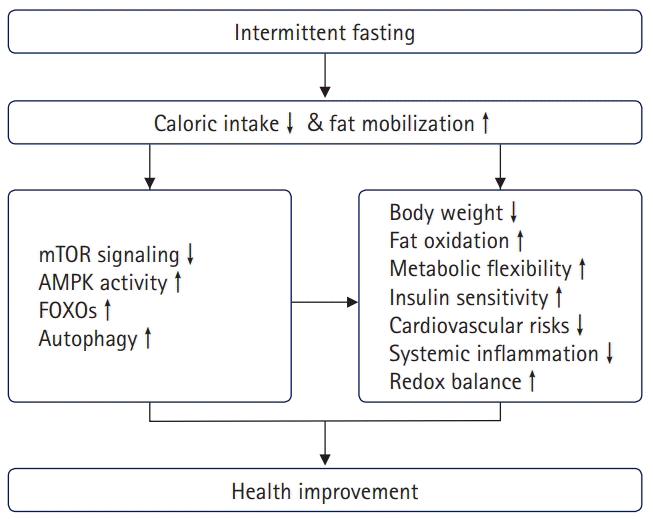
Weight reduction is the primary mechanism underlying the beneficial effects of IF. As shown in the results from CR, weight reduction per se reduces fasting plasma insulin levels, cardiovascular risk factors, and body inflammatory status by regulating metabolic signaling pathways, including those involving forkhead box O (FOXO), mechanistic target of rapamycin (mTOR), AMP‐activated protein kinase (AMPK), and autophagy [11]. During the fed state, signaling pathways for nutrient sensing and cellular growth (e.g., mTOR) are activated. Stress-responsive signaling pathways (e.g., FOXO and AMPK) are activated by fasting, resulting in the protection from cell damage and inhibition of cell proliferation [23,24].
An additional mechanism of IF is the metabolic switch between fed and fasting states. Fasting, especially repetitive fasting, induces organisms to shift their metabolic phase, which improves metabolic conditions and extends health expectancy [18]. de Cabo and Mattson [25] reported that fasting optimizes cellular use of fuel sources, favoring ketone bodies and fatty acids over glucose, which ameliorates the blunting of metabolic flexibility observed in obesity and type 2 diabetes mellitus (T2DM) [26] and improves mitochondrial function [27]. Furthermore, fasting activates autophagy and defense mechanisms against oxidative and metabolic stress and suppresses inflammation [28-31]. These effects of IF are similar to those of aerobic exercise [32,33]. Fasting induces glucose and amino acid deprivation, stimulating AMPK activity and suppressing mTOR signaling, which are important nutrient-sensing signaling pathways. These changes inhibit FOXO-dependent gene transcription, resulting in the induction of autophagy and oxidative defense mechanisms [34] (Fig. 1). During IF, the body activates pathways for rejuvenation and repair [19].
Overall, the general effects of IF are beneficial in terms of physiological functions; however, some participants who participated in IF trials experienced reductions in bone density and lean body mass [35-38]. To preserve lean body mass, a protein-rich diet and accompanying resistance training are recommended [39].
Go to : 
The human body has precise regulatory mechanisms to maintain body weight homeostasis [40]. However, chronic caloric excess results in excessive accumulation of fat tissue (obesity) and is associated with various metabolic alterations, such as hypertension, diabetes, dyslipidemia, cardiovascular disease, and even some types of cancer [41,42]; controlling caloric intake may reverse these metabolic alterations. Although the cause of obesity is multifactorial, dietary management is a primary approach to control body weight; thus, optimal dietary treatment should consider safety, efficacy, nutritional balance, cultural acceptance, and economic status [43]. Many studies have indicated that IF is an effective and acceptable intervention in obese subjects, including obese adolescents [2,17,44,45].
As described above, CR has long been applied as a primary treatment modality for obesity; recently, IF has appeared as an alternative dietary intervention to CR because dieters feel that IF is a more tolerable method than CR [4,17-19,46].
According to previous clinical trials [10,47] and reviews [14,48-50], IF (4–24 weeks) induces body weight reductions of 4% to 10% in overweight individuals [51-53]. The varying degree of body weight reduction depends on the dietary pattern, dietary duration, diet composition, sex, and genetic response. Although some studies have shown greater body fat reductions with IF than with CR [14,54], the majority of these studies have shown equivalent effects on reductions in body weight and fat mass following IF or CR in overweight or obese individuals [33,55].
Considering IF patterns, weight reduction effects are more profound in ADF (average weight reduction of 0.75 kg per week) than in 5:2 IF (average weight reduction of 0.25 kg per week) because of different negative energy balances [56,57].
There were mixed results with regard to lean body mass; several systemic reviews suggested that regular IF decreased fat-free mass more than CR [50,58]. However, clinical trials [17,57] and other reviews [47,49,58] indicated that IF and CR produced similar loss of lean body mass. Moreover, Harvie et al. [14] found that IF participants maintained a higher lean mass than CR participants. Stekovic et al. [18] reported that ADF for 6 months did not reduce fat-free mass or bone density in healthy nonobese subjects. However, a recent study showed that IF may be associated with a higher rate of weight regain following cessation of the 6-month weight reduction phase than CR in patients with complex obesity [59]. Further studies are needed to assess the ability to lose weight without regaining it.
The basic mechanism of the weight loss by IF involves reduced caloric intake. However, the change in body weight caused by 40% CR and 2-day IF per week was not simply double that caused by 20% CR and 1-day IF per week in mice, suggesting an additional physiological response to fasting [35]. Another mechanism of weight reduction may be associated with the shift from glucose to fatty acid metabolism resulting from the fasting-induced elevation in fat mobilization and utilization [25,31]. The reduction in insulin, an anabolic hormone, by IF may also be responsible for the reduction in body fat mass [60].
Go to : 
Obesity is currently a leading cause of the development of T2DM, which results from insulin resistance and oxidative stress induced by elevated blood glucose and free fatty acid levels [61]. Weight reduction directly improves insulin resistance and reverses these metabolic alterations [22,62].
Although there are some inconsistent results, most studies indicate that IF decreases insulin concentration and the homeostasis model assessment for insulin resistance [51,63-65].
An IF trial for 12 months in T2DM patients showed that body weights, glycated hemoglobin levels, and fasting levels of glucose and insulin were reduced with IF [47,66] and that the insulin-lowering effect was greater with IF than with CR [67,68]. In the diabetic state, IF reduces the plasma concentrations of glucose and insulin and elevates adiponectin levels [2,69]. Although the primary mechanism of these effects is mediated by weight loss, metabolic switching following repeated feeding and fasting, and reductions in inflammatory cytokines, reactive oxygen species, and cholesterol may be involved [22].
The effect of IF on glucose homeostasis is different in nondiabetic and nonobese subjects. According to a study by Stekovic et al. [18], 4 weeks of ADF treatment did not change insulin sensitivity despite significant body weight reductions in healthy nonobese individuals, suggesting that these participants were already in an insulin-sensitive state. Moreover, Heilbronn and Ravussin [70] showed that 3 weeks of ADF treatment suppressed glucose tolerance in nonobese women, while insulin sensitivity was improved in nonobese men. Clayton et al. [71] also reported that 1 day of severe CR impaired glycemic control in young lean men. This suggests different responses to IF in healthy weight and obese subjects. Overall, IF has benefits on the diabetic state; however, the risks of hypoglycemia, malnutrition of proteins and vitamins, and dehydration have also been reported [22,47,72]. Careful monitoring and adjustment of medication regimens are needed for patients at risk.
In an animal study, IF improved glucose homeostasis by preserving pancreatic β-cell mass through the autophagy-lysosomal pathway in diet-induced obese diabetic mice [73].
Go to : 
A feature of metabolic syndrome is the clustering of metabolic alterations such as abdominal obesity, insulin resistance, dyslipidemia, atherosclerosis, and hypertension, which are associated with the risk of cardiovascular diseases [45,74,75].
Randomized clinical trials have indicated that IF improves lipid profiles related to weight reduction [5-10]. Klempel et al. [8] showed that ADF decreased total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. Trepanowski et al. [10] also observed the cardioprotective effects of 6-month ADF in obese adults. These cardioprotective effects of IF have also been observed in obese adolescents [17] and nonobese subjects. Moro et al. [38] observed lipid profile enhancement (increased high-density lipoprotein [HDL] and decreased LDL levels) in a 2-month trial of IF in healthy men. Stekovic et al. [18] observed that a 6-month trial of ADF in healthy nonobese subjects lowered levels of total cholesterol, LDL, very-low-density lipoprotein (VLDL), and triglycerides compared with the corresponding levels in controls. Moreover, the authors observed a decrease in systolic blood pressure, which is consistent with studies conducted on obese subjects [14,48].
The effects of IF on HDL levels have been varied. Meng et al. [76] did not show any change in HDL cholesterol. In contrast, Bhutani et al. [77] observed an elevation in HDL cholesterol.
The mechanisms of improving cardiovascular disease risks by IF may result from obesity control, improved lipid profiles, elevated adiponectin levels [69], and a suppressed inflammatory state [78,79]. Additionally, increased hepatic fatty acid oxidation in the fasting state results in reduced hepatic accumulation of triglycerides, which sequentially decreases the hepatic production of VLDL and plasma levels of VLDL [22,80]. Adiponectin is an adipose tissue-derived adipokine that has anti-atherosclerotic and anti-inflammatory effects [81]. Adiponectin was shown to be elevated by IF intervention in obese subjects [70]. In an animal study, IF protected the heart from oxidative damage via activation of antioxidant defenses [82].
Go to : 
Macrophages infiltrate hypertrophied adipose tissue and produce proinflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) [83-85], which induce insulin resistance and atherosclerosis and are linked to low-grade systemic inflammation [86,87]. The plasma concentrations of these inflammatory cytokines parallel the degree of obesity and are positively correlated with insulin resistance [85]. Systemic inflammation is linked to the pathogenesis of T2DM, cardiovascular diseases, and some types of cancers [88,89]. Thus, systemic inflammatory markers can predict the development of these metabolic disorders [85].
Body weight reduction decreases adipose tissue macrophages [90], reduces proinflammatory cytokines [83,88,91-95], and improves insulin resistance and systemic inflammatory status [86]. Several clinical trials have shown that IF intervention improves inflammatory status in obese subjects and is associated with reductions in plasma levels of IL-6, TNF-α, C-reactive protein (CRP), and interferon-γ [60,96]. Wang et al. [42] revealed that IF intervention decreased CRP levels without changes in IL-6 and TNF-α compared with the corresponding levels in controls in a systematic review of 18 randomized controlled trials. However, there have been some inconsistent studies. Liu et al. [96] reported that IF increased macrophage infiltration in adipose tissue by fasting in overweight or obese women, which may be associated with elevated adipose tissue lipolysis. Schübel et al. [33] did not observe any changes in IL-6 and TNF-α levels after 12 weeks of IF in randomized controlled trials with obese women.
Go to : 
IF has emerged as an alternative dietary intervention to CR, with equivalent benefits in body weight reduction, improvements in glucose homeostasis and lipid profiles, and anti-inflammatory effects. The beneficial effects of IF are mediated by reductions in body weight. Weight loss per se improves insulin resistance, cardiovascular risks, and systemic inflammatory status because obesity functions as a common pathophysiology of these metabolic alterations, the “common soil hypothesis” [97]. Moreover, the insulin-lowering effect is greater in IF than in CR resulting from fasting physiology, in which repetitive metabolic switching between feeding and fasting states improves the metabolic flexibility that is blunted in obesity and T2DM.
Although the general effects of IF are beneficial in terms of metabolic functions, some participants who participated in IF trials experienced reductions in bone density and lean body mass. Thus, careful monitoring, a protein-rich diet, and accompanying isometric resistance training are recommended to preserve lean body mass and bone density.
Go to : 
Notes
Author contributions
Conceptualization: YWK; Data curation: YWK; Project administration: DKS, YWK; Writing-original draft: DKS, YWK; Writing-review & editing: DKS, YWK.
Go to : 
References
1. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017; 377:13–27.
2. Malinowski B, Zalewska K, Węsierska A, Sokołowska MM, Socha M, Liczner G, et al. Intermittent fasting in cardiovascular disorders: an overview. Nutrients. 2019; 11:673.
3. Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017; 39:36–45.
4. Duregon E, Pomatto-Watson L, Bernier M, Price NL, de Cabo R. Intermittent fasting: from calories to time restriction. Geroscience. 2021; 43:1083–92.
5. Hill JO, Schlundt DG, Sbrocco T, Sharp T, Pope-Cordle J, Stetson B, et al. Evaluation of an alternating-calorie diet with and without exercise in the treatment of obesity. Am J Clin Nutr. 1989; 50:248–54.
6. Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009; 90:1138–43.
7. Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011; 10:119.
8. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. Eur J Clin Nutr. 2013; 67:783–5.
9. Varady KA, Dam VT, Klempel MC, Horne M, Cruz R, Kroeger CM, et al. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci Rep. 2015; 5:7561.
10. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017; 177:930–8.
11. Hwangbo DS, Lee HY, Abozaid LS, Min KJ. Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients. 2020; 12:1194.
12. Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011; 12:e593–601.
13. Anton S, Leeuwenburgh C. Fasting or caloric restriction for healthy aging. Exp Gerontol. 2013; 48:1003–5.
14. Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013; 110:1534–47.
15. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014; 164:302–11.
16. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017; 39:46–58.
17. Jebeile H, Gow ML, Lister NB, Mosalman Haghighi M, Ayer J, Cowell CT, et al. Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity. J Nutr. 2019; 149:1189–97.
18. Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019; 30:462–76.
19. Hu D, Xie Z, Ye Y, Bahijri S, Chen M. The beneficial effects of intermittent fasting: an update on mechanism, and the role of circadian rhythm and gut microbiota. Hepatobiliary Surg Nutr. 2020; 9:597–602.
20. Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E. Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity (Silver Spring). 2020; 28(Suppl 1):S29–37.
21. Rouhani MH, Azadbakht L. Is Ramadan fasting related to health outcomes? A review on the related evidence. J Res Med Sci. 2014; 19:987–92.
22. Grajower MM, Horne BD. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients. 2019; 11:873.
23. Kenyon C. A conserved regulatory system for aging. Cell. 2001; 105:165–8.
24. Brandhorst S, Harputlugil E, Mitchell JR, Longo VD. Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res Rev. 2017; 39:68–77.
25. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019; 381:2541–51.
26. Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008; 295:E1009–17.
27. Abdellatif M, Sedej S. Cardiovascular benefits of intermittent fasting. Cardiovasc Res. 2020; 116:e36–8.
28. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014; 20:1006–17.
29. Chapnik N, Genzer Y, Froy O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J Nutr Biochem. 2017; 40:116–21.
30. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018; 362:770–5.
31. Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018; 19:63–80.
32. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018; 26:254–68.
33. Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018; 108:933–45.
34. Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022; 23:56–73.
35. Zhang G, Deighan A, Raj A, Robinson L, Donato HJ, Garland G, et al. Intermittent fasting and caloric restriction interact with genetics to shape physiological health in mice. Genetics. 2022; 220:iyab157.
36. Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res. 2016; 31:40–51.
37. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985). 2007; 102:634–40.
38. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016; 14:290.
39. Real-Hohn A, Navegantes C, Ramos K, Ramos-Filho D, Cahuê F, Galina A, et al. The synergism of high-intensity intermittent exercise and every-other-day intermittent fasting regimen on energy metabolism adaptations includes hexokinase activity and mitochondrial efficiency. PLoS One. 2018; 13:e0202784.
40. Moon KH, Park SY, Kim YW. Obesity and erectile dysfunction: from bench to clinical implication. World J Mens Health. 2019; 37:138–47.
41. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444:840–6.
42. Wang X, Yang Q, Liao Q, Li M, Zhang P, Santos HO, et al. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. 2020; 79-80:110974.
43. Koliaki C, Spinos T, Spinou Μ, Brinia ΜE, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018; 6:73.
44. Sundfør TM, Svendsen M, Tonstad S. Intermittent calorie restriction-a more effective approach to weight loss? Am J Clin Nutr. 2018; 108:909–10.
45. Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021; 106:64–79.
46. Chausse B, Vieira-Lara MA, Sanchez AB, Medeiros MH, Kowaltowski AJ. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS One. 2015; 10:e0120413.
47. Carter S, Clifton PM, Keogh JB. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res Clin Pract. 2019; 151:11–9.
48. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011; 35:714–27.
49. Davis CS, Clarke RE, Coulter SN, Rounsefell KN, Walker RE, Rauch CE, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016; 70:292–9.
50. Roman YM, Dominguez MC, Easow TM, Pasupuleti V, White CM, Hernandez AV. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: a systematic review and meta-analysis of randomized controlled trials. Int J Obes (Lond). 2019; 43:2017–27.
51. Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring). 2014; 22:2524–31.
52. Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015; 73:661–74.
53. Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring). 2016; 24:1874–83.
54. Alhamdan BA, Garcia-Alvarez A, Alzahrnai AH, Karanxha J, Stretchberry DR, Contrera KJ, et al. Alternate-day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta-analysis. Obes Sci Pract. 2016; 2:293–302.
55. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019; 11:2442.
56. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention. A scientific statement from the American Heart Association. Circulation. 2017; 135:e96–121.
57. Tinsley GM, Horne BD. Intermittent fasting and cardiovascular disease: current evidence and unresolved questions. Future Cardiol. 2018; 14:47–54.
58. Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with type II diabetes: a randomised trial. Int J Obes Relat Metab Disord. 2003; 27:797–802.
59. Antoni R, Johnston KL, Steele C, Carter D, Robertson MD, Capehorn MS. Efficacy of an intermittent energy restriction diet in a primary care setting. Eur J Nutr. 2020; 59:2805–12.
60. Zubrzycki A, Cierpka-Kmiec K, Kmiec Z, Wronska A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J Physiol Pharmacol. 2018; 69:663–83.
61. Kim YW, Moon JS, Seo YJ, Park SY, Kim JY, Yoon JS, et al. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem Biophys Res Commun. 2012; 420:462–6.
62. Morales-Suarez-Varela M, Collado Sánchez E, Peraita-Costa I, Llopis-Morales A, Soriano JM. Intermittent fasting and the possible benefits in obesity, diabetes, and multiple sclerosis: a systematic review of randomized clinical trials. Nutrients. 2021; 13:3179.
63. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007; 42:665–74.
64. Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013; 12:4.
65. Harvie MN, Sims AH, Pegington M, Spence K, Mitchell A, Vaughan AA, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016; 18:57.
66. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. 2018; 1:e180756.
67. Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018; 16:371.
68. Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018; 16:507–47.
69. Bhutani S, Klempel MC, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver Spring). 2010; 18:2152–9.
70. Heilbronn LK, Ravussin E. Calorie restriction extends life span: but which calories? PLoS Med. 2005; 2:e231.
71. Clayton DJ, Burrell K, Mynott G, Creese M, Skidmore N, Stensel DJ, et al. Effect of 24-h severe energy restriction on appetite regulation and ad libitum energy intake in lean men and women. Am J Clin Nutr. 2016; 104:1545–53.
72. Liu K, Liu B, Heilbronn LK. Intermittent fasting: what questions should we be asking? Physiol Behav. 2020; 218:112827.
73. Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy. 2017; 13:1952–68.
74. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–9.
75. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002; 288:2709–16.
76. Meng H, Zhu L, Kord-Varkaneh H, O Santos H, Tinsley GM, Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020; 77:110801.
77. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring). 2013; 21:1370–9.
78. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013; 5:1218–40.
79. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016; 24:1612–9.
80. Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010; 20:124–37.
81. Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009; 4:44–9.
82. Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, et al. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med. 2010; 48:47–54.
83. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–808.
84. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–49.
85. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014; 105:141–50.
86. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995; 95:2409–15.
87. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116:1793–801.
88. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–30.
89. Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015; 24:283–307.
90. Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006; 290:E961–7.
91. Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, et al. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006; 136:343–52.
92. Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010; 59:2817–25.
93. Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016; 11:e0154003.
94. Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of Moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016; 23:591–601.
95. Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 2017; 66:392–406.
96. Liu B, Hutchison AT, Thompson CH, Lange K, Heilbronn LK. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract. 2019; 13:408–15.
97. Stern MP. Diabetes and cardiovascular disease. The "common soil" hypothesis. Diabetes. 1995; 44:369–74.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download