Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) has been established as a treatment modality for superficial esophageal squamous cell carcinoma (ESCC). Long-term follow-up data are lacking in Western countries. The aim of this study was to analyze long-term survival in a Western center.
Methods
Patients undergoing ESD for ESCC were included. The analysis was performed retrospectively using a prospectively collected database.
Results
R0 resection rate was 96.7% (59/61 lesions in 58 patients). Twenty-seven patients (46.6%) fulfilled the curative resection criteria (M1/M2) (group A), 11 patients (19.0%) had M3 lesions without lymphovascular invasion (LVI) (group B), and 20 patients (34.5%) had lesions with submucosal invasion or LVI (group C). Additional treatment was recommended after non-curative resection. It was not performed in 20/31 patients (64.5%), mainly because of comorbidities (75%). Twenty-nine out of 58 (50.0%) patients died during a mean follow-up of 3.7 years. Death was related to ESCC in 17.2% (5/29) of patients. The disease-specific survival rate after curative resection was 100%. Overall survival rates after 5 years were 61.5%, 63.6% and 28.1% for groups A, B, and C, respectively. The overall survival was significantly worse after non-curative resection (p=0.038).
In 2020, 604,100 new cases of esophageal cancer (EC) and 544,076 deaths from EC were reported worldwide. EC is the eighth most common cancer and the sixth most common cause of cancer-related deaths. Incidence rates range from 12.3/10,000 in Eastern Asia to 4.1/100,000 in Western Europe.1 Esophageal squamous cell carcinoma (ESCC) is the predominant subtype of EC worldwide, accounting for more than 90% of all ECs in Asia. In contrast, esophageal adenocarcinoma (EAC) has become the most frequent subtype in many Western countries.2 Reflecting the geographical differences, studies on EAC have been conducted predominantly by Western endoscopists, whereas studies on ESCC have been mainly published by Asian groups.3,4 Endoscopic resection (ER) offers a minimally invasive curative treatment option for EC diagnosed in the early stages with negligible risk of lymph node metastasis (LNM). ER is effective for superficial lesions of both histological subtypes. Endoscopic submucosal dissection (ESD) for superficial ESCC was shown to be superior to other resection techniques in terms of en bloc resection and recurrence rates.5 Therefore, ESD is strongly recommended for ER of ESCC by the current Japanese and European guidelines.6,7 The European Society of Gastrointestinal Endoscopy defines ER of ESCC as curative when R0 resection is confirmed, invasion depth is limited to the M1/M2 layer, and lymphovascular invasion (LVI) is histologically ruled out.6 The Japanese guidelines define these lesions as an “absolute indication” for ER. These resections are classified as “curative”, and follow-up without further treatment is recommended.7,8 Well-differentiated lesions invading the lamina muscularis mucosae (MM) or the superficial submucosal layer (submucosal invasion ≤200 µm) have a low risk of LNM when LVI is ruled out and R0 resection is confirmed. The European Society of Gastrointestinal Endoscopy describes ER as “curative in the majority of cases” and recommends a multidisciplinary discussion balancing the risk of further therapy against the risk of LNM individually.6 Japanese guidelines classify these lesions as “relative indications” for ER. These guidelines recommend a comprehensive evaluation of additional treatment (AT) for lesions invading the MM and recommend AT (surgery or chemoradiation) for lesions with LVI and for lesions invading the submucosal layer.7,8 ER techniques and treatment algorithms have improved over the last decade. However, follow-up data of ESD for ESCC are scarce, especially outside of Asia. Most ESCCs are caused by risk factors, such as alcohol and tobacco consumption, and patients with ESCC often have significant comorbidities. Our study aimed to evaluate the long-term prognosis of Western patients after ESD for ESCC.
The single-center uncontrolled study was conducted in a German referral center (Department of Gastroenterology, University Hospital Augsburg, Augsburg, Germany). All the patients who underwent ESD for ESCC between August 2007 and September 2021 were included in this study. All patients undergoing ESD in the department were enrolled in a local database after obtaining informed consent. The data were retrospectively analyzed using this database. The study was conducted in accordance with the principles of Good Clinical Practice and the ethical guidelines of the 1975 Declaration of Helsinki. This study was approved by the ethics committee of the Ludwig-Maximilian-University, Munich, Germany (study ID: 22-0046).
• Patients who underwent ESD for superficial esophageal squamous cell neoplasia.
Written informed consent was obtained for the procedure after detailed information about ESD and alternative treatment strategies (surgery, chemoradiation) was provided. For circumferential lesions, the additional risk of postinterventional stricture was discussed, and the treatment strategy was based on the patients’ individual decisions.
• Patients who showed endoscopic ultrasound findings of invasion depth >T1 and/or suspected LNM for ESCC.
• Patients with concomitant malignant disease without curative treatment option.
Curative resection was diagnosed when histopathological analysis confirmed pT1a cancer invading the M1/M2 level and LVI was ruled out (group A). Non-curative resections were assigned to group B (pT1a invading the M3 level without LVI) or group C (pT1b and any lesion with LVI). Patients with a follow-up period of more than 6 months were included in the follow-up analysis and analyzed separately within the different groups.
The primary outcome parameter was overall survival after ESD. Secondary outcome parameters were disease-specific survival and procedural characteristics (en bloc resection rate, curative resection rate, complications, and AT after ESD).
Diagnostic endoscopy and ESD procedure were performed using a videogastroscope (GIF-H180, GIF-HQ190, or GIF-EZ1500; Olympus Medical Systems, Tokyo, Japan). White light imaging (WLI) and narrow-band imaging (NBI) were used for all lesions. Lugol staining was used additionally when the demarcation was unclear with WLI and NBI. The lesion's morphology was described according to the Paris classification.9 The proximal margin of the lesion, the length, and the degree of its circumferential extension were recorded. There was no restriction on lesion size or degree of the circumferential extension. The staging procedure included endoscopic ultrasonography and computed tomography of the neck, chest, and upper abdomen. Biopsies confirming squamous cell neoplasia (high-grade dysplasia or ESCC) were available for all lesions before ESD.
ESD was performed in a standardized manner as described previously (Fig. 1).3 Insufflation was performed using carbon dioxide. The procedures were performed under general anesthesia. When the course was uneventful, patients stayed in the hospital for 48 to 96 hours after ESD, depending on the endoscopist’s decision. ESD was performed by three endoscopists experienced in ESD (AP, AE, and HM). Each endoscopist performed at least 25 gastric and 25 rectal ESDs prior to the first esophageal ESD. According to current guidelines, anticoagulants except aspirin were stopped before ER and were restarted 5-7 days after the procedure depending on the endoscopist’s decision.10 All patients received proton pump inhibitors for three months (pantoprazole 40 mg twice daily for six weeks and once daily for another six weeks).
The resection specimens were minimally stretched and fixed onto a cork with needles. After formalin fixation, all specimens were cut transversely to the long axis. The specimens were cut into thin parallel sections of 2 mm thickness. To optimize orientation, the margins were marked with color before cutting. Embedding in a 90-degree orientation enabled excellent evaluation of the slides regarding the involvement of the lamina MM and the depth of invasion. Routine staining included hematoxylion and eosin and immunohistochemistry with D2-40. Additional staining was performed using desmin, CD56, and p40 individually.
When the resected ulcer exceeded 75% of the circumference, stricture prevention was performed. The regimen for stricture prevention was modified during the study period. From 2007 to 2012, preventive balloon dilation was performed twice weekly within the first week after ESD and weekly thereafter until complete healing of the resection ulcer and the patients were free from dysphagia. From January 2013 to April 2014, prednisolone was administered orally over eight weeks (40 mg daily, with a 5-mg dose reduction weekly) based on Japanese studies.11 From May 2014, we introduced a modified regimen using oral prednisolone (starting dose 50 mg; standard tapering over eight weeks using 50/40/30/25/20/15/10/5 mg). Tapering and duration of administration were individualized according to the ulcer healing process (assessed endoscopically) in the first tapering period and before stopping steroids.12
Complications were defined as bleeding, perforation, stricture, and death. Severe intraprocedural bleeding was noted as a complication, leading to premature termination of ESD. Delayed bleeding was defined as the occurrence of hematemesis and/or melena after ESD. Bleeding was classified as major when the hemoglobin drop exceeded 2 g/L.13 Intraprocedural perforation was defined as an obvious defect in the muscularis propria during ESD. Delayed perforation was diagnosed when post-interventional endoscopy confirmed a defect of the muscularis propria, or radiologic imaging showed extravasation of the contrast medium. Stricture was defined as a complication when patients complained of dysphagia.
Follow-up endoscopy using WLI and NBI was scheduled at 3, 6, and 12 months after ESD and annually thereafter. Endoscopic ultrasonography and computed tomography of the neck, chest, and upper abdomen were recommended after 6 and 12 months and annually thereafter when the resection was judged as non-curative and the patients’ condition allowed ATs in case of recurrence.
Variables were described as counts and percentages or mean and standard deviation. The chi-square test or Fisher exact test was used to compare the different patient groups. Continuous variables were compared using ANOVA or the Kruskal-Wallis test. For each group, a Kaplan-Meier curve of the overall survival and disease-specific survival was estimated, and differences between these groups were tested using a log-rank test. The statistical analysis was performed using R ver. 4.1.0 (R Core Team 2021, Vienna, Austria). All tests were two-sided, and a p-value <0.05 was considered significant.
Over a 14-year period, ESD was performed in 60 patients. Fifty-seven patients presented with one ESCC and another three with two synchronous ESCCs, resulting in a total of 63 ESDs. Histopathological analysis did not confirm ESCC in two resection specimens, despite previous biopsies showing ESCC in one of these patients and high-grade dysplasia in the other. These two patients were excluded from further analyses. In the remaining 58 patients, histology confirmed curative resection in 27 patients (46.6%) (group A). Eleven patients (19.0%) were classified into group B and 20 patients (34.5%) into group C (Fig. 2). Group C included three patients with synchronous lesions which were resected during a single procedure. The synchronous lesions fulfilled the curative resection criteria, but the patients were classified into group C according to the more aggressive lesion. The patient characteristics, comorbidities, and indications for diagnostic endoscopy are shown in Table 1. The lesions characteristics are summarized in Table 2.
After excluding two patients without neoplasia in the ESD specimen, the procedure characteristics were analyzed for 61 ESDs (Table 3). ESD was performed successfully for all lesions. R0 resection was confirmed in 59/61 ESCCs (96.7%), while two specimens (3.3%) showed R1 resection at the horizontal margin despite the use of Lugol staining. Strictures were seen in 7/61 resections (11.5%). The stricture rate was 85.7% (6/7) after circumferential resection and 9.1% (1/11) after resection of 76% to 99% of the circumference. All the strictures were endoscopically managed. Bleeding, perforation, or procedure-related mortality were not observed.
Twenty-seven patients (46.6%) were followed-up after curative resection without AT (group A). AT was recommended for 11 patients in group B and 20 patients in group C. AT was performed in 1/11 patients (9.1%) in group B and in 10/20 patients (50.0%) in group C. AT was not performed in 20/31 patients in both the groups (64.5%). AT was not performed because of comorbidities in 15 patients (previous chemoradiation of the mediastinum, n=5; chronic obstructive pulmonary disease, n=4; ischemic heart disease, n=3; liver cirrhosis, n=3), and patient refusal in another five (Fig. 2).
Fifty-eight patients were included in the follow-up analysis and analyzed separately for different groups. The three groups were comparable in terms of patient characteristics, comorbidities, and R0 resection (Table 4). The AT rate was significantly higher in group C (p<0.001). Local recurrence was observed in 4/20 patients (20.0%) in group C (three patients without AT and one patient after chemoradiation). Metastatic recurrence was diagnosed in one patient in group B (liver metastases 29 months after ESD and additional chemoradiation of an M3 L0 ESCC with poor differentiation) and in another three patients in group C (pulmonary metastases 15 months after ESD without AT, lymph node metastases 7 months after ESD without AT, liver metastases 40 months after ESD, and additional chemoradiation) (Fig. 2). In patients who underwent R1 resection of the horizontal margin, no recurrence occurred. Metachronous lesions were not observed during the follow-up.
A total of 29/58 (50.0%) patients died during follow-up. Five deaths were related to recurrent ESCC (four patients with metastatic disease and one patient with local recurrence who refused further treatment and nutrition). Twenty-four deaths were related to other causes (other malignancies, n=9; cardiovascular disease, n=8; pulmonary disease, n=3; liver cirrhosis, n=1; others, n=3). Mortality rates were 37.0%, 63.6%, and 60.0% in group A, B and C, respectively (p=0.180). Mean follow-up periods were 3.9 years, 5.8 years, and 2.5 years, respectively. Disease specific survival was 100% after curative resection and significantly worse after non-curative resection (p=0.038). Overall survival was comparable after curative and non-curative resections (p=0.015). Disease-specific survival and overall survival is shown in Figure 3.
ESD is recommended for ER of superficial ESCC according to Asian and European guidelines.6,7 However, data are mainly restricted to Asian publications, and data from Western regions are scarce. In particular, the long-term follow-up is unknown in Western patients. Therefore, we analyzed the follow-up after ESD for ESCC in a European referral center. Asian studies have demonstrated a high rate of technical success for ESD in superficial ESCC. En bloc resection and complete resection rates were 95.1% and 89.4% in a meta-analysis that included 15 Asian studies.14 Smaller European studies described R0 resection in 80% to 98%.15-17 In our study, we confirmed an R0 resection rate of 96.7%. ESD seems to be an ideal ER method for superficial ESCC and has gained acceptance worldwide.
High R0 resection rates in contrast to much lower rates of curative resection, which have been reported as 46.2% to 71.4% in European studies.16,17 ER of ESCC is defined as curative for M1/M2 lesions without LVI.6 Japanese guidelines define these lesions as “absolute indication” for ER.7,8 Lesions invading the MM (M3) or the superficial submucosal layer (submucosal invasion ≤200 µm) have a low risk of LNM when LVI is ruled out. Japanese guidelines classify these lesions as “relative indications” for ER. A comprehensive evaluation of AT is recommended for lesions invading the MM without LVI and AT (surgery or chemoradiation) is recommended for lesions with LVI and for lesions invading the submucosal layer.7,8
In our study, 46.6% of patients fulfilled the curative resection criteria, 19.0% were M3 lesions without LVI, and 34.5% were lesions with submucosal invasion and/or LVI after ESD. Asian studies have reported higher rates of curative resection. Recently, Iwai et al.18 reported 659 patients who underwent ESD for ESCC in ten Japanese centers over a 9-year-period. The absolute indication criteria was fulfilled by 68.9%, relative indication criteria was fulfilled by 12.3%, and only 4.7% were shown to be lesions with deep submucosal invasion or LVI. One possible reason for the low curative resection rates in Western studies may be the rareness of superficial ESCC, causing difficulties in the pretherapeutic assessment of histopathological features such as invasion depth and LVI. In a large German multicenter study, which included 306 esophageal ESDs, only 51 were ESCCs.17 Another difference between Asian and Western countries is screening endoscopies of the upper gastrointestinal tract, which are routinely performed in Asian countries and may allow for diagnosis of ESCC in earlier stages. In our study, the majority of ESCCs were diagnosed during follow-up endoscopy in high-risk patients with previous SCCs or esophageal varices. Pre-interventional assessment of superficial ESCC is crucial and must be improved in Western countries. The pre-interventional work-up should include image-enhanced endoscopy and/or Lugol chromoendoscopy to define the extent of lesions and endoscopic ultrasound to define the invasion depth and rule out LNM. Furthermore, magnifying endoscopy with NBI is recommended to assess the depth of invasion before treatment.19,20 A large Japanese study reported a positive predictive value of 93% for invasion of the epithelium/lamina propria, 65% for invasion of the MM or superficial submucosal invasion, and 77% for deep submucosal invasion.21 The data on NBI have been published during the last few years and were not available during the first half of our study.
In our study, AT had to be recommended in 53.4% of patients when resection was judged as non-curative. AT was performed in 1/11 (9.1%) patients with M3 lesions without LVI and 10/20 (50.0%) patients with lesions invading the submucosal layer or showing LVI. In contrast, AT was not performed in 20/31 patients in both groups (64.5%). AT was not performed due to comorbidity (75%) and patient refusal (25%). This data shows the clinical problems of European patients who present with significant comorbidity, mostly associated with a history of alcohol and tobacco abuse. In the present study, 62.1% of the patients reported alcohol abuse, 58.6% reported tobacco abuse, and a substantial proportion of patients presented with a history of previous SCCs (ear, nose, throat cancer or ESCC), cardiovascular disease, chronic obstructive pulmonary disease, or liver cirrhosis.
During long-term follow-up, 29/58 (50.0%) of the patients died. Five deaths were related to recurrent ESCC, whereas 24 were related to other causes (predominantly other malignancies in 31.0% and cardiovascular disease in 24.1%). ESCC-related deaths were observed in the MM (9.1%) and the submucosal invasion/LVI (20%) groups. No ESCC-related death was observed after curative resection. The low rate of ESCC-related deaths in the M3 group confirms the Japanese data, which allows follow-up without AT for those patients. A large Japanese study reported recurrence rates of 2.3% for M3 lesions and 4.3% for lesions invading the superficial submucosa (≤200 µm). Risk factors for recurrence without AT were LVI (hazard ratio [HR], 5.61), positive vertical margin (HR, 4.55), and submucosal-invasion >200 µm (HR, 3.25).22
Corresponding to the literature, disease-specific survival in our study was 100% after curative ER and significantly worse after non-curative resection (p=0.038). In contrast to the excellent disease-specific survival, the 5-year overall survival was 61.5% after curative resection and significantly worse after resection of submucosal invasion/LVI lesions (28.1%) (p=0.025). The prognosis of our patients was mainly unrelated to ESCC and mostly determined by the underlying comorbidities caused by alcohol and tobacco consumption. A recent study from Korea described a 5-year survival rate of 60.2% for patients with a Charlson comorbidity index of >2. Similar to our results, secondary malignancies were the most common cause of death (75%).23
Our study confirms the efficacy of ESD for superficial ESCC in terms of en bloc and R0 resection in a Western referral center. However, the rate of non-curative resections remains substantial and more than one-third of patients show histopathological high-risk features (submucosal invasion, LVI) despite R0 resection. Early diagnosis and pre-interventional stratification regarding endoscopic versus non-endoscopic treatment need to be improved in the Western world. The long-term prognosis of superficial ESCC is limited in Western patients and is mainly determined by secondary malignancies and cardiovascular comorbidities associated with alcohol and tobacco consumption.
REFERENCES
1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer;2020. [cited 2021 Jul 11]. Available from: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=6&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D.
2. Arnold M, Ferlay J, van Berge Henegouwen MI, et al. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020; 69:1564–1571.
3. Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015; 47:113–121.
4. Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005; 3(7 Suppl 1):S67–S70.
5. Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010; 72:255–264.
6. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:829–854.
7. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019; 16:25–43.
8. Ishihara R, Arima M, Iizuka T, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020; 32:452–493.
9. Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005; 37:570–578.
10. Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016; 48:385–402.
11. Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011; 73:1115–1121.
12. Probst A, Ebigbo A, Märkl B, et al. Stricture prevention after extensive endoscopic submucosal dissection of neoplastic Barrett’s esophagus: individualized oral steroid prophylaxis. Gastroenterol Res Pract. 2019; 2019:2075256.
13. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
14. Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014; 59:1862–1869.
15. Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010; 71:715–721.
16. Lorenzo D, Barret M, Leblanc S, et al. Outcomes of endoscopic submucosal dissection for early oesophageal squamous cell neoplasia at a Western centre. United European Gastroenterol J. 2019; 7:1084–1092.
17. Fleischmann C, Probst A, Ebigbo A, et al. Endoscopic submucosal dissection in Europe: results of 1000 neoplastic lesions from the German Endoscopic Submucosal Dissection Registry. Gastroenterology. 2021; 161:1168–1178.
18. Iwai N, Dohi O, Yamada S, et al. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: a multi-center cohort study. Surg Endosc. 2022; 36:2279–2289.
19. Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. 2020; 53:142–166.
20. Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017; 14:105–112.
21. Katada C, Tanabe S, Wada T, et al. Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J Gastrointest Cancer. 2019; 50:292–297.
22. Hatta W, Koike T, Takahashi S, et al. Risk of metastatic recurrence after endoscopic resection for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa: a multicenter retrospective study. J Gastroenterol. 2021; 56:620–632.
23. Kim GH, Na HK, Ahn JY, et al. Long-term outcomes and factors affecting the survival of patients with mucosal esophageal squamous cell carcinoma. Gut Liver. 2021; 15:705–712.
Fig. 1.
Endoscopic submucosal dissection procedure of an esophageal squamous cell carcinoma in the proximal esophagus. (A) White light endoscopy. (B) Narrow-band imaging. (C) Resection ulcer after endoscopic submucosal dissection. (D) Histology showing esophageal squamous cell carcinoma restricted to the mucosa (M1) without lymphovascular invasion (absolute indication for endoscopic resection; hematoxylin and eosin stain, ×40).
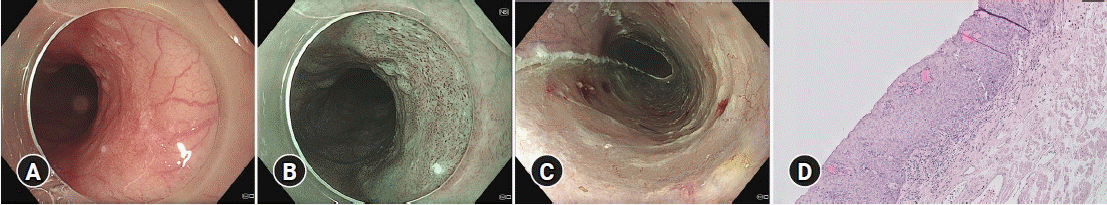
Fig. 2.
Patients' inclusion and additional treatment after endoscopic submucosal dissection. ESD, endoscopic submucosal dissection; SM, submucosal invasion; AT, additional treatment.
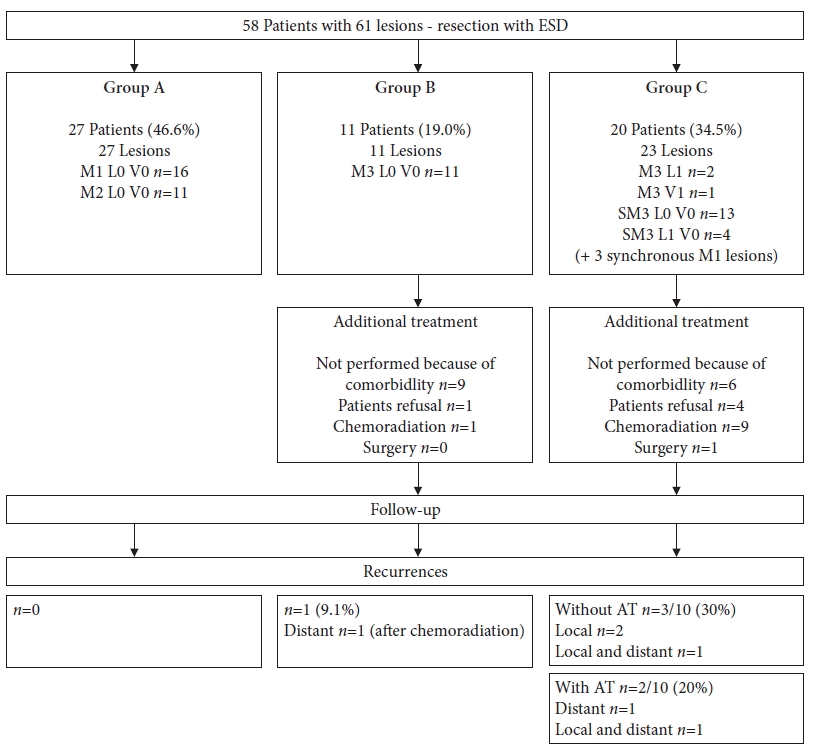
Fig. 3.
Kaplan-Meier survival after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. (A) Overall survival for the different groups A, B, C. (B) Disease specific survival for the different groups A, B, C. (C) Overall survival after curative resection versus non-curative resection. (D) Disease specific survival after curative resection versus non-curative resection.
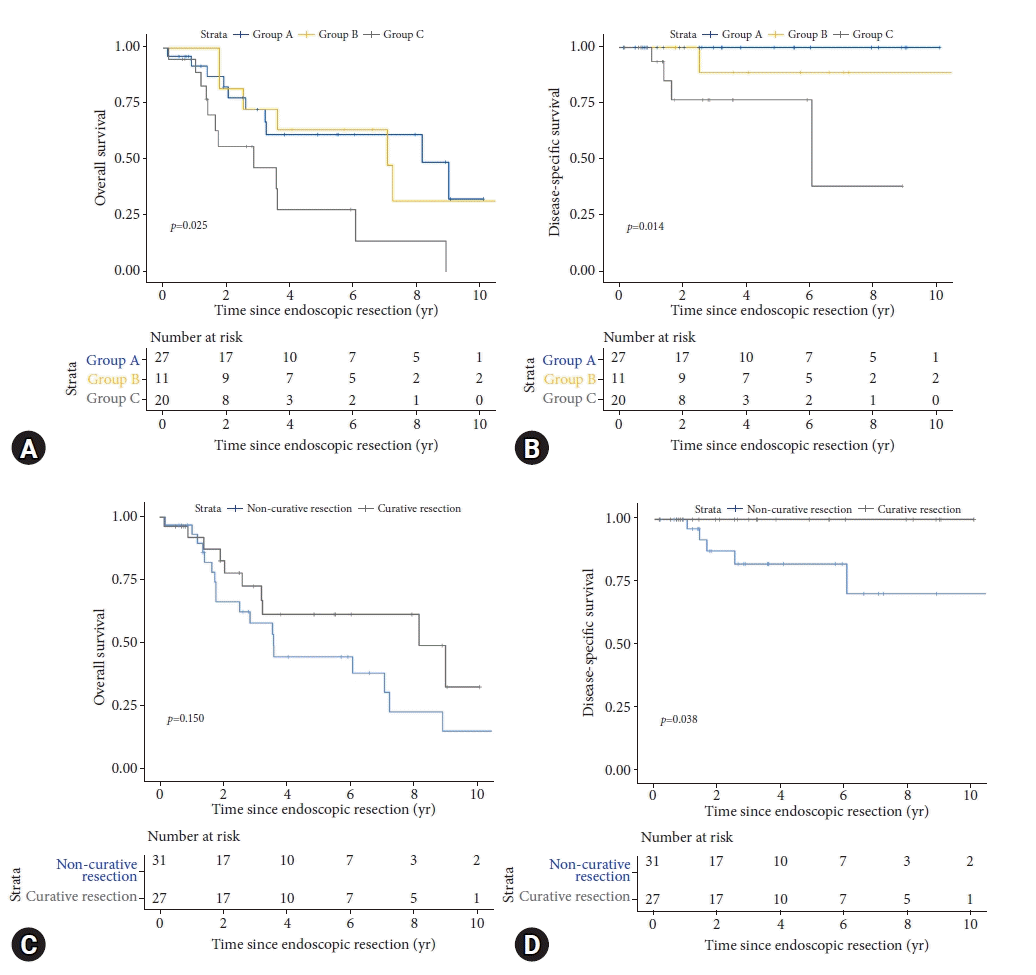
Table 1.
Patients characteristics
Table 2.
Lesions characteristics (61 resected lesions)
Table 3.
Procedure characteristics (61 resected lesions)
| Characteristic | Value |
|---|---|
| Resection specimen (cm2) | 10.9±6.4 |
| Resection rates | |
| R0 resection | 59 (96.7) |
| R1 resection horizontal margin | 2 (3.3) |
| R1 resection vertical margin | 0 (0) |
| Curative resection (M1/M2 without LVI) | 30 (49.2) |
| Non-curative resection | 31 (50.8) |
| M3 without LVI | 11 (18.0) |
| pT1b or any LVI | 20 (32.8) |
| Circumferential extension of the resection ulcer after ESD | |
| ≤50% | 12 (19.7) |
| 50%–75% | 31 (50.8) |
| 76%–99% | 11 (18.0) |
| Circumferential | 7 (11.5) |
| Complications | |
| Stricture | 7 (11.5)a) |
| Bleeding | 0 (0) |
| Perforation | 0 (0) |
Table 4.
Follow-up results in 58 patients with a minimum follow-up of six months




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download