INTRODUCTION
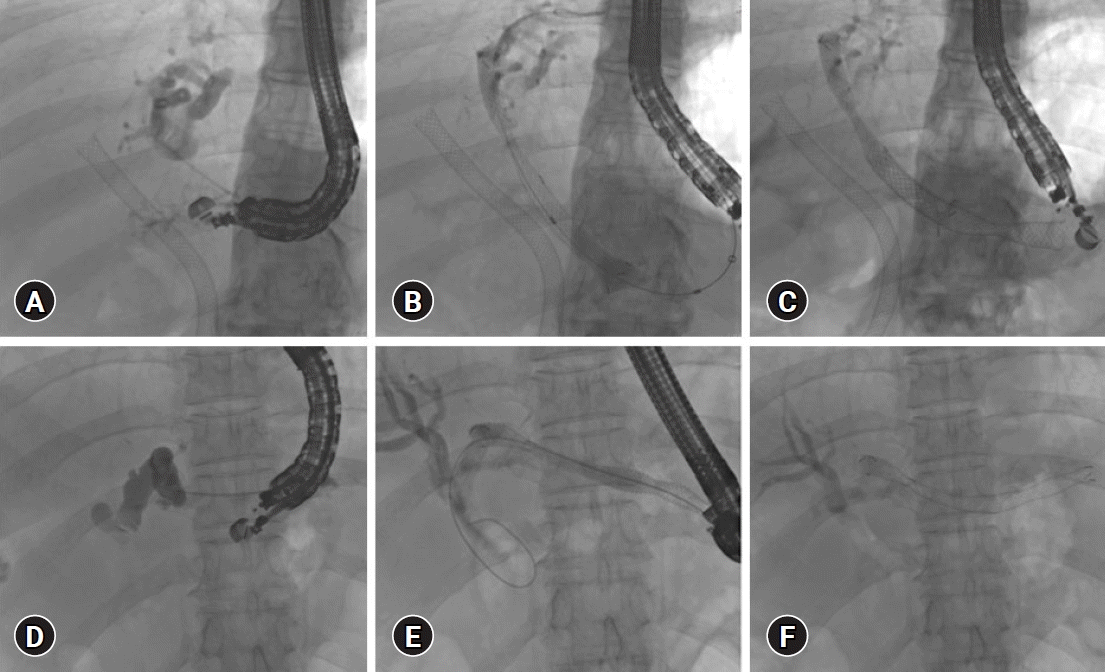 | Fig. 1.Endoscopic ultrasound (EUS) guided hepatico-gastrostomy (HGS). (A–C) EUS-HGS for isolated left intra-hepatic dilation post-transpapillary self-expanding metal stent (SEMS) placement: (A) Left hepatic duct puncture, (B, C) SEMS placement through EUS-HGS fistula created by EUS guidance. (D–F) EUS-HGS for communicating hilar block: (D) Puncture of left hepatic duct, (E, F) SEMS placement over the guidewire. |
EVOLUTION OF EUS-GUIDED INTERVENTIONS FOR MHBO WITH INACCESSIBLE PAPILLA
BILATERAL BILIARY DRAINAGE WITH EUS-HGS
Table 1.
| Study | Year | n | Mode of drainage | Technical success (%) | Clinical success (%) | Adverse events |
|---|---|---|---|---|---|---|
| Bories et al.9 | 2007 | 11 (8 malignant) | HGS | 90.9 | 90.9 | Biloma: 1 |
| Cholangitis: 1 | ||||||
| Tissue ingrowth: 1 | ||||||
| Stent migration: 1 | ||||||
| Panpimanmas et al.12 | 2013 | 10 (cholangiocarcinoma) | HGS | 80 | 70 | Stent malposition: 1 |
| Stent migration into stomach: 2 | ||||||
| Ogura et al.14 | 2014 | 10 High-grade hilar block | HGS: 8 | 100 | 90 | None |
| HDS: 2 | ||||||
| Ogura et al.21 | 2015 | 11 Right hepatic duct obstruction | HDS: 4 | 100 | 100 | None |
| Bridging stent: 7 | ||||||
| Reimão et al.15 | 2014 | 9 MHBO | HGS | 77.7 | 77.7 | Sepsis and death: 1 |
| Failed drainage: 1 | ||||||
| Abdominal pain: 2 | ||||||
| Caillol et al.16 | 2019 | 12 With hilar obstruction and inaccessible papilla | HGS+bridging technique | 100 | 83 | Fulminant sepsis and death: 1 |
| Abdominal pain: 3 | ||||||
| Salvage percutaneous drainage: 1 | ||||||
| Ma et al.22 | 2020 | 35 Isolated right duct obstruction (25 malignant) | All HDS | 97.1 | 80 | 20% |
| Minaga et al.5 | 2017 | 30 MHBO, failed re-intervention | HGS: 28 | 96.6 | 75.9 | Mild peritonitis: 3 |
| HDS: 2 | Stent dysfuction: 7 | |||||
| Moryoussef et al.28 | 2017 | 15 MHBO with inaccessible papilla | HGS | 94 | 72.2 | 16.7% |
| Winkler et al.29 | 2021 | 20 MHBO | 16 Initial drainage (2 HGS alone, 14 HGS in combination with other technique) | 100 | 95 | Early complication: 35% |
| 4 Re-intervention | Mortality: 5% | |||||
| Kitamura et al.31 | 2022 | 15 MHBO, all failed transpapillary re-intervention | EUS-guided re-intervention (EUS-BD) | 100 | 86.7 | Biliary peritonitis: 13.3% |
| Yamamura et al.33 | 2022 | 14 MHBO, post multiple SEMS | Molting technique: novel stent delivery system with a dilation function (EndoSheather; Piolax Medical Devices, Kanagawa, Japan) | 92.8 | 92.8 | 2 Paients with failed re-intervention: rescue EUS-BD with uncovered SEMS |
| Kongkam et al.34 | 2021 | 36 (19 CERES vs. 17 PTBD) | ERCP+HGS: 17 | CERES: 84 | CERES: 78.9 | CERES 26.3% vs. PTBD 35.5% |
| ERCP+HDS: 1 ERCP+HGS+HDS: 1 | PTBD: 100 | PTBD: 76.5 | Median time to re-intervention: CERES 92 days vs. PTBD 40 days (p=0.006) | |||
| ERCP+HGS+HDS: 1 |
EUS, endoscopic ultrasound; EUS-BD, EUS-guided biliary drainage; MHBO, malignant hilar biliary obstruction; HGS, hepatico-gastrostomy; HDS, hepatico-duodenostomy; SEMS, self-expanding metal stent; CERES, combined endoscopic retrograde cholangiopancreatography and EUS-BD; PTBD, percutaneous trans-hepatic biliary drainage.
EUS-HDS IN SEGREGATED RIGHT INTRA-HEPATIC DUCT
RE-INTERVENTION AFTER EUS-BD
GUIDEWIRE MANIPULATION
CERES
RE-INTERVENTION AFTER MULTIPLE METALLIC STENTS PLACEMENT FOR MHBO
CERES VERSUS BILATERAL PTBD FOR MHBO
STENT-IN-STENT AND MULTI-STENT METHOD
NOVEL INTERVENTIONS THROUGH EUS-GUIDED HGS
EUS-BD IN CONJUNCTION WITH INTERVENTIONAL RADIOLOGY
PROCEDURAL CONSIDERATIONS OF EUS-GUIDED INTERVENTIONS IN MHBO
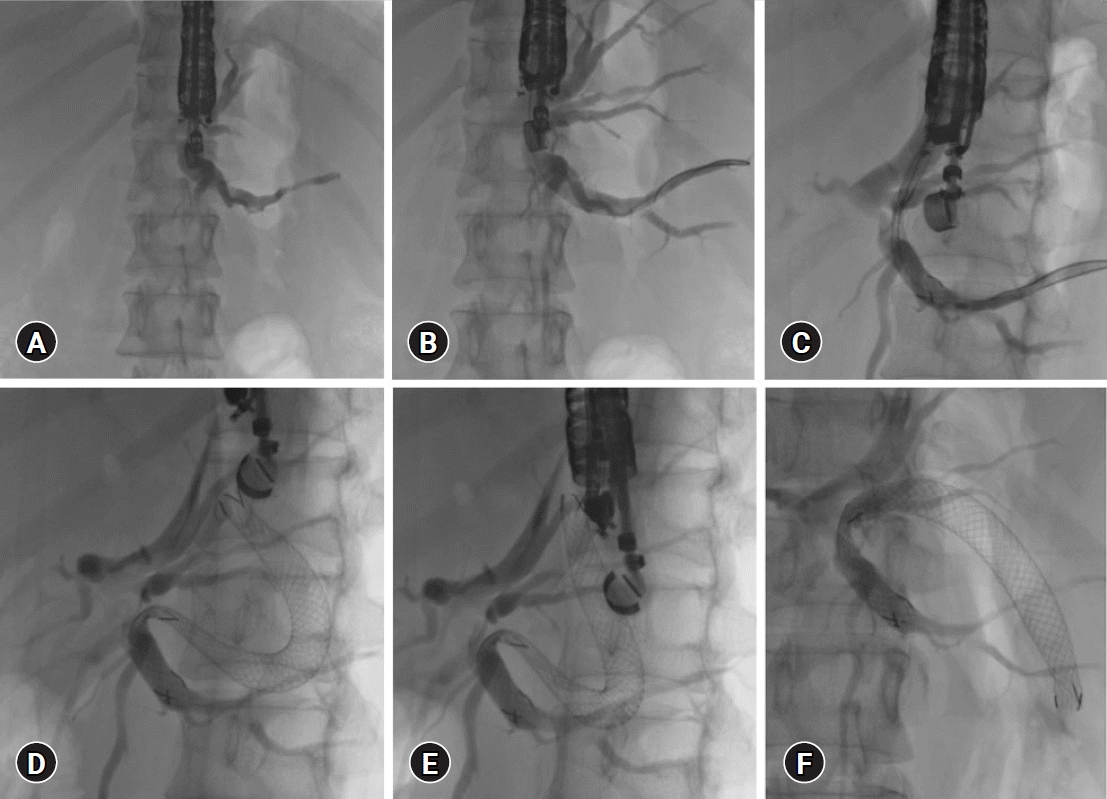 | Fig. 2.Steps of endoscopic ultrasound-guided hepatico-gastrostomy. (A) Puncture site selection, segment 3 duct is preferred over segment 2. (B) Segment 3 duct punctured with 19-G needle and 0.025 stiff guidewire. (C) Deploying the gastric end of the stent within the scope. (D, E) Post-self-expanding metal stent deployment. (F) Final self-expanding metal stent position. |
COMPLICATIONS
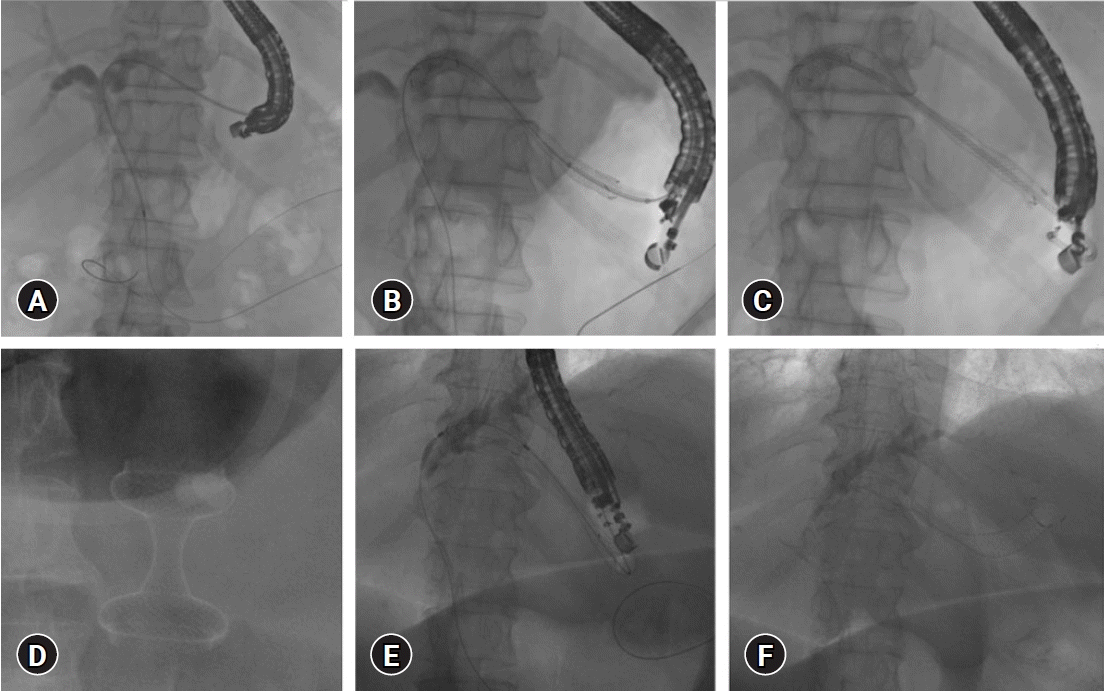 | Fig. 3.Anchoring plastic stent placement within self-expanding metal stent (SEMS) during endoscopic ultrasound (EUS) guided hepatico-gastrostomy (HGS) (A–C), combined EUS-guided gastrojejunostomy (D), and HGS for malignant obstruction with inaccessible papilla due to duodenal obstruction (E, F). (A) EUS-guided antegrade placement of guidewire across papilla. (B) SEMS placement through HGS fistula. (C) Anchoring plastic stent placement through SEMS. (D) EUS-guided gastrojejunostomy with a hot AXIOS stent. (E) EUS-guided antegrade guidewire passage for inaccessible papilla due to duodenal stenosis. (F) Post SEMS placement. |
LIMITATIONS
CONCLUSIONS
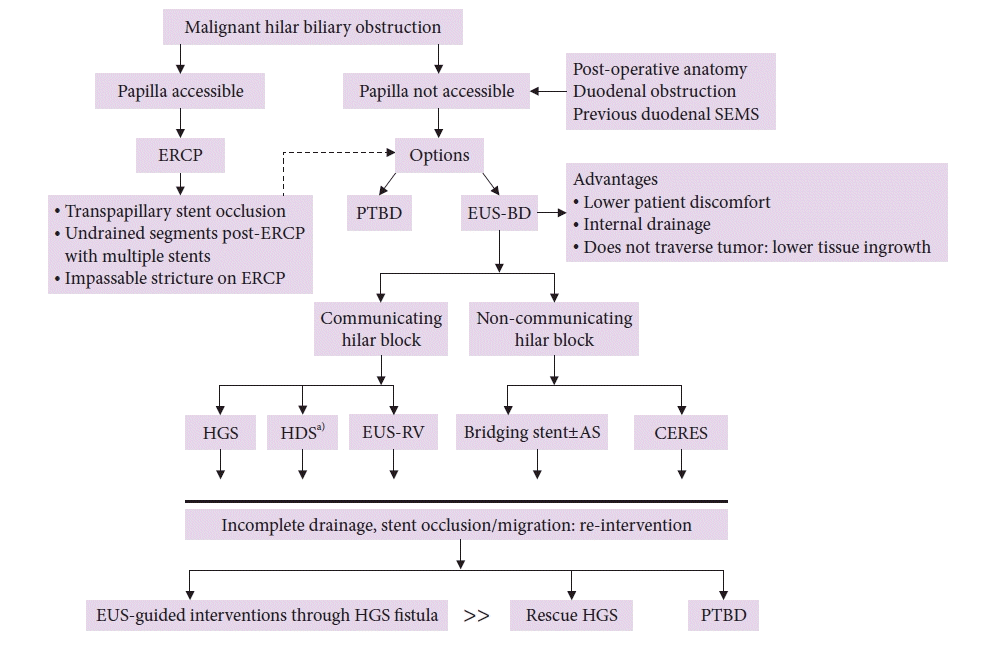 | Fig. 4.Approach to biliary drainage for malignant hilar biliary obstruction. SEMS, self-expanding metal stent; ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous trans-hepatic biliary drainage; EUS-BD, endoscopic ultrasound-guided biliary drainage; HGS, hepatico-gastrostomy; HDS, hepatico-duodenostomy; RV, rendezvous; AS, antegrade stenting; CERES, combined ERCP and EUS-BD.
a)Also for isolated right intra-hepatic duct dilation.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download