Abstract
Background/Aims
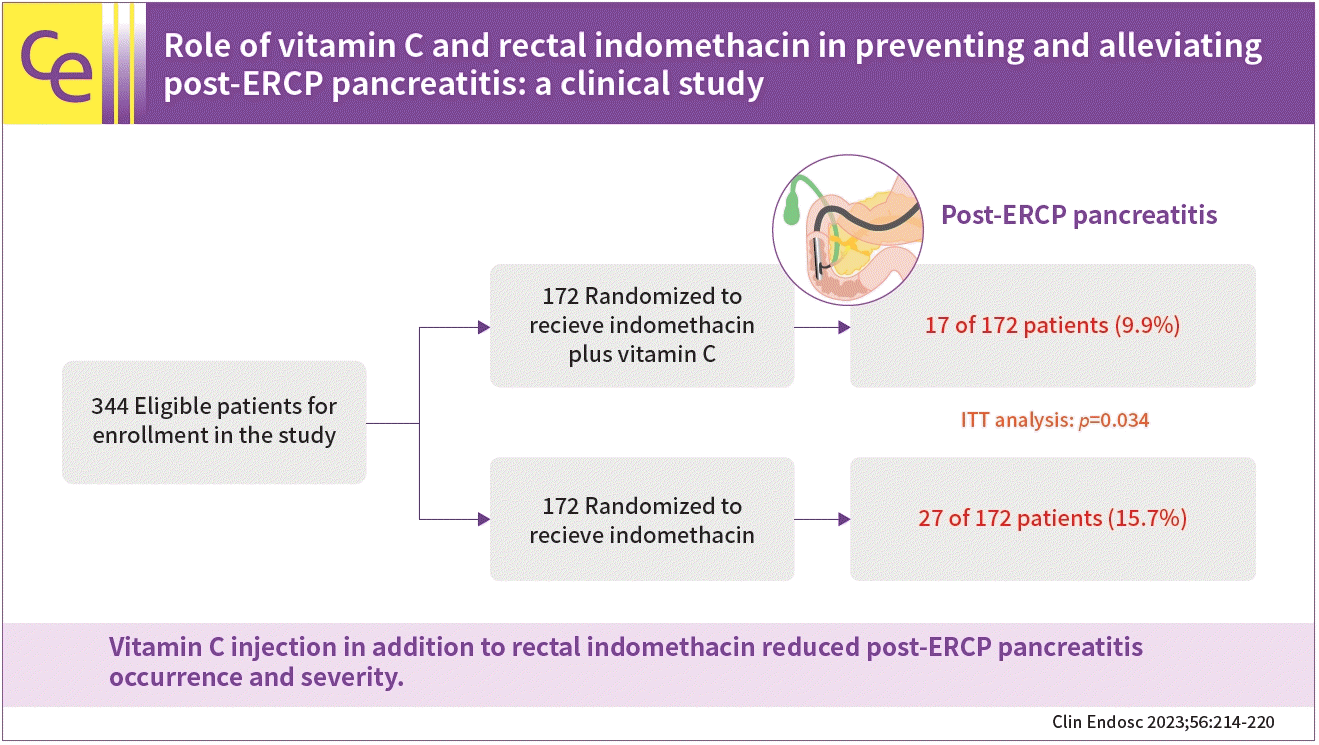
This study aimed to determine whether vitamin C in addition to indomethacin decreases the occurrence and severity of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) occurrence and severity.
Methods
This randomized clinical trial included patients undergoing ERCP. The participants were administered either rectal indomethacin (100 mg) plus an injection of vitamin C (500 mg) or rectal indomethacin (100 mg) alone just before ERCP. The primary outcomes were PEP occurrence and severity. The secondary amylase and lipase levels were determined after 24 hours.
Results
A total of 344 patients completed the study. Based on intention-to-treat analysis, the PEP rates were 9.9% for indomethacin plus vitamin C plus indomethacin and 15.7% for indomethacin alone. Regarding the per-protocol analysis, the PEP rates were 9.7% and 15.7% in the combination and indomethacin arms, respectively. There was a remarkable difference between the two arms in PEP occurrence and severity on intention-to-treat and per-protocol analyses (p=0.034 and p=0.031, respectively). The post-ERCP lipase and amylase concentrations were lower in the combination arm than in the indomethacin alone arm (p=0.034 and p=0.029, respectively).
Go to : 
Endoscopic cannulation of the ampulla of Vater was first performed more than 60 years ago and has provided a means of obtaining comprehensive radiographic images of the pancreatobiliary ducts. The introduction of endoscopic retrograde cholangiopancreatography (ERCP) has been a dominant advance in medicine.1 While ERCP has improved diagnostic and treatment modalities for pancreatobiliary diseases, complications following ERCP persist and may have an important effect on patient morbidity and mortality. Common post-ERCP complications include pancreatitis, bleeding, cholecystitis, infections, and intestinal perforation.2 Post-ERCP pancreatitis (PEP) is the most serious unfavorable effect of this procedure. PEP is a type of acute pancreatitis (AP). The incidence of PEP is variable, but it seems 2% to 16%, with a higher occurrence observed in high-risk patients. Some patient- and procedure-associated risk factors increase the risk of PEP.3 Although its exact underlying mechanism is not well known, various theories have been proposed. However, for mechanical, chemical, hydrostatic, enzymatic, infectious, allergic, and thermal mechanisms that are suspected to trigger PEP, evidence demonstrates that one or more of them are responsible for PEP or influence its severity.4 Several pharmacologic agents have been tried to avoid or alleviate PEP, such as anti-inflammatories (indomethacin and diclofenac),5 antioxidants (allopurinol and N-acetylcysteine [NAC]),6,7 antibiotics,8 and epinephrine.9 Rectal non-steroidal anti-inflammatory drugs are the cornerstone of PEP prevention according to the European Society of Gastrointestinal Endoscopy guidelines.10
Oxidative stress (OS) is considered involved in AP pathogenesis. Bopanna et al.11 measured the OS and antioxidant position of patients with AP. Participants diagnosed with idiopathic recurrent AP were enrolled in the study, and markers of OS (including 4-hydroxynonenol, malondialdehyde, and serum superoxide dismutase) and antioxidant position (including ferric reducing the ability of plasma, glutathione peroxidase, and vitamin C level) were determined in the inactive phase and during AP. They reported that 4-hydroxynonenol levels were significantly elevated in patients with idiopathic recurrent AP versus healthy controls (p=0.03), even higher during an episode of AP (p=0.03). In addition, antioxidant concentrations were lower in idiopathic recurrent AP than in healthy volunteers.
Vitamin C is a remarkable antioxidant in mammals that can influence the outcomes of situations such as AP in which OS plays an important role.12 In one study, vitamin C levels were determined in 30 healthy volunteers versus 29 with a diagnosis of AP and 27 with other acute abdominal problems.13 A disproportionate decrease in serum vitamin C level compared to the prevailing level of vitamin C was a characteristic of AP in this study. Siriwardena et al.14 performed a randomized trial of a combination of intravenous antioxidant (NAC, selenium, vitamin C) therapy in 43 patients with predicted severe AP. Combined antioxidant or placebo was administered via an infusion device. They reported that relative plasma concentrations of antioxidants increased, while markers of OS, such as C-reactive protein, fell in the active arm during the study. Considering the lack of data regarding the role of vitamin C in the inhibition or alleviation of PEP, the present trial aimed to determine whether the combination of vitamin C and indomethacin could reduce PEP occurrence and severity (main outcome) and whether combination therapy could control the increase in pancreatic enzymes after ERCP (secondary outcome).
Go to : 
The present clinical trial was conducted in the gastrointestinal procedures ward of Talighani Hospital affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran, from May to November 2021.
The inclusion criteria were as follows: (1) age 18 to 85 years with suitable indications for diagnostic or therapeutic ERCP due to suspected pancreatobiliary disorders; and (2) written informed consent for enrollment in the trial. Patients were excluded from the trial if they had any of the following: (1) need for pancreatobiliary stent replacement; (2) need for emergency ERCP; (3) diagnosis of AP 3 days prior to ERCP; (4) history of hypersensitivity reaction to vitamin C, aspirin, and any non-steroidal anti-inflammatory drugs; (5) currently pregnant or nursing; (6) creatinine clearance <30 mL/min; (7) history of gastrointestinal bleeding or asthma; (8) post-ERCP and receiving any medications that might influence PEP occurrence or severity, such as epinephrine and octreotide; and (9) history of previous biliary/pancreatic sphincterotomy.
The present randomized open-label trial enrolled a total of 405 eligible patients indicated for ERCP. At baseline, each patient’s complete medical history and blood samples were obtained. The patients were randomized at a 1:1 ratio to receive either rectal indomethacin 100 mg (Tolidaru Pharmaceutical Co., Tehran, Iran) plus an injection of vitamin C 500 mg (Daroupakhsh Pharmaceutical Co., Tahran, Iran) or rectal indomethacin at the same dose alone. Participants were randomized using an individual center-based computer-generated block randomization random number list. Participants took their allocated medications at least 30 minutes before undergoing ERCP, which was performed by two experienced endoscopists who performed more than 250 ERCP procedures annually. Both specialists worked at this center for at least 5 years. All participants were sedated using the same protocol of midazolam (0.06 mg/kg) and morphine (15 mg for patients aged less than 60 years or 10 mg for patients aged >60 years). Post-ERCP blood specimens were obtained approximately 24 hours after ERCP to determine the pancreatic enzyme (amylase and lipase) concentrations. All serum pancreatic enzyme levels were determined using an automated chemistry analyzer (Cobas 8000 C702; Roche Hitachi, Basel, Switzerland). The reference normal values for pancreatic enzymes were 20 to 80 IU/L (amylase) and 9 to 60 IU/L (lipase). After ERCP, all patients were monitored closely in the gastroenterology and liver ward of Talighani Hospital for the manifestation of probable abdominal pain and the occurrence of pancreatitis at least 24 hours after ERCP.
In this trial, PEP was classified as mild, moderate, or severe based on the revised Atlanta classification. Mild PEP was defined as two of the following: pain consistent with AP; amylase or lipase more than three times the normal limit; characteristic imaging findings; and no organ dysfunction or adverse events. Moderate PEP was defined as transient organ failure within 48 hours or local or systemic adverse events without persistent organ failure. Severe PEP was defined as persistent single or multiple organ failure >48 hours or present or persistent systemic inflammatory response syndrome.15
Post-ERCP blood specimens were obtained for the determination of serum amylase and lipase levels 24 hours after ERCP. The results are presented as mean±standard deviation. The data were analyzed using the IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA), and p-values <0.05 were considered statistically significant. To demonstrate a decrease in the PEP occurrence rates from 14% to 5% because of vitamin C addition by using the chi-square test with an alpha error of 0.05 in a two-sided test and a power of 0.80, at least 165 participants in each arm were required.
The Ethics Committee of Shahid Beheshti University of Medical Sciences approved this study (no. IR.SBMU.PHARMACY.REC 1399.375). The study was recorded at the Iranian Clinical Trial Registry (IRCT 20121021011192N11).
Go to : 
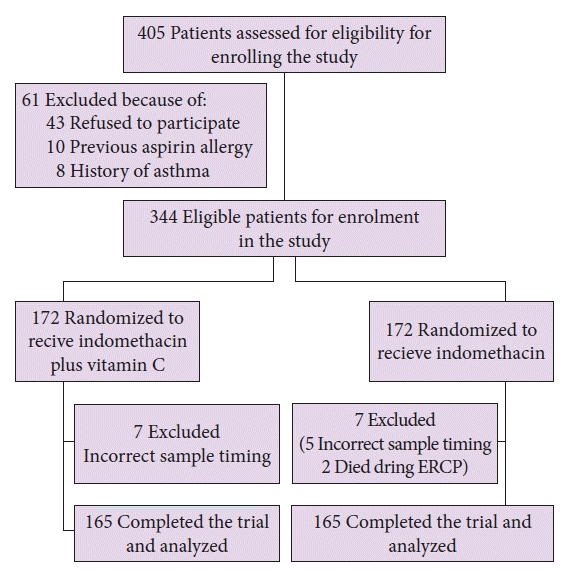
A total of 405 participants were prescreened; of them, 61 were excluded. Thus, a total of 344 patients were enrolled in the present trial and randomized. Finally, 330 participants completed the study per the protocol. A flowchart of the patient enrollment process is shown in Figure 1.
Mean age, sex, mean body mass index, concentration of pancreatic enzymes at baseline, indication for ERCP, and procedure difficulty (based on the American Society of Gastroenterology and Endoscopy grading system)16 are shown in Table 1. No remarkable intergroup differences were noted in baseline amylase and lipase levels, mean age, sex distribution, ERCP indications, and procedural difficulty.
Baseline clinicodemographic data in the participants
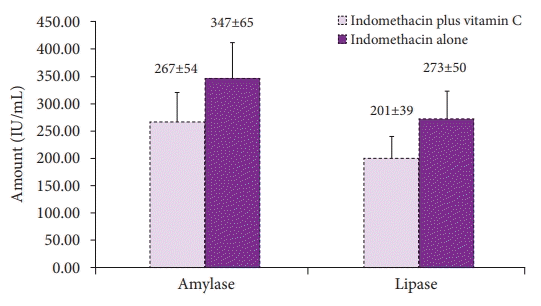
A total of 344 participants undergoing ERCP were included in the intention-to-treat (ITT) analysis, while 330 participants were included in the per-protocol (PP) analysis. A total of 43 of 344 patients in the ITT analysis and 42 of 330 patients in the PP analysis had PEP (12.5% and 12.7%, respectively). Table 2 shows the PEP rate and severity of the indomethacin plus vitamin C and the indomethacin groups. PEP occurrence was lower in the vitamin C plus indomethacin arm than in the indomethacin alone arm, and the analysis showed an obvious difference between the two arms in the ITT and PP analyses (p=0.034 and p=0.031, respectively). The mean concentrations of pancreatic enzymes in the two arms after ERCP are shown in Figure 2. Serum amylase levels were lower in the combination arm than in the indomethacin-alone arm. Similar results were observed for serum lipase levels between the two arms. Statistical analysis showed a significant intergroup difference in the post-ERCP concentrations of serum amylase and lipase (p=0.029 and p=0.034, respectively).
Rate and severity of post-ERCP pancreatitis among two groups
Regarding probable adverse drug reactions, all participants in the indomethacin plus vitamin C group and the indomethacin alone group tolerated the medications well, and no patients dropped out of either study.
Go to : 
PEP is a complication whose exact mechanism remains controversial. Damage to the pancreatic acinar cells starts a complex flood of events that contain elevated levels of reactive oxygen species, resulting in the oxidation of proteins and lipids and upset of the pancreatic membrane.17 Acinar cells produce and release proteases that facilitate digestion in response to cholecystokinin stimulation after food consumption. These enzymes become active in the small intestine only, but in pathologic conditions, changes occur in acinar cell signaling, leading to premature arousal of these enzymes, the secretion of inflammatory and vascular biomarkers, inhibition of acinar cell release, and arousal of apoptosis pathways. The activation of nuclear factor of kappa light chain enhancer B (NF-κB), which serves as a chief regulator of inflammation in PEP, leads to the synthesis of inflammatory biomarkers such as interleukin-6, interleukin-1B, cyclooxygenase 2, and transforming growth factor-β.18
As OS plays an expected role in the pathogenesis of PEP, agents that can ameliorate it would improve outcomes.17 Levels of vitamin C, an important endogenous antioxidant in humans, are reduced in patients with AP. Bonham et al.19 evaluated whether vitamin C levels continue to decline after hospital admission according to AP severity. They measured vitamin C levels in 62 and 23 patients with mild and severe AP, respectively, on the day of enrollment as well as on days 2 and 5. Plasma levels of vitamin C in patients with AP were remarkably lower than those in healthy participants on days 0, 2, and 5 (p<0.001), and this was more prominent in those with severe AP. In addition, there was a decline in serum vitamin C levels from day 0 to day 2 in patients with mild (p<0.001) or severe (p=0.005) AP and from day 2 to day 5 in patients with severe AP (p=0.023).
The positive role of vitamin C in pancreatitis was reported in previous studies, although no reports have described the effect of vitamin C on PEP. Previous meta-analyses investigated the ability of some antioxidants (e.g., allopurinol, NAC, selenium) to inhibit or alleviate PEP.20,21 Although one trial examined the combination effect of vitamin C, NAC, and selenium in PEP,14 the present study is the first to evaluate the effect of vitamin C plus indomethacin on PEP occurrence or severity. The European Society of Gastrointestinal Endoscopy 2020 guideline suggests delivering diclofenac or indomethacin immediately before or after ERCP to prevent PEP.10 Therefore, the present trial was designed to determine whether the injection of a single dose of vitamin C, in addition to rectal indomethacin, could reduce PEP rate and/or severity. It is important to decrease the effect of confounding factors in trials that evaluate the efficacy of pharmacological agents against PEP rate and severity. Köseoğlu et al.22 conducted a retrospective study of 666 patients undergoing ERCP. They reported that female sex, a lower common bile duct diameter, and plastic biliary stent placement were risk factors for PEP in patients with bile duct stones. In our trial, the arms were matched with respect to sex, indication for ERCP, and difficulty performing ERCP.
In our trial, the injection of 500 mg of vitamin C and administration of 100 mg of rectal indomethacin showed an inhibitory effect against PEP. To assess the efficacy and safety of antioxidants for inhibiting or alleviating PEP, an antioxidant was administered solely against placebo in the majority of clinical trials. Therefore, in a meta-analysis, Gooshe et al.21 indicated that, in contrast to AP, antioxidant therapy does not confer protection against PEP. In contrast to most previous trials, the present trial compared the additive effect of vitamin C with indomethacin to that of indomethacin alone (instead of placebo). Some patients in each study arm was diagnosed with PEP (9.7% in the vitamin C plus indomethacin arm and 15.7% in the indomethacin alone arm). The statistical analysis showed that the PEP rate was remarkably higher in the indomethacin-alone arm than in the combination arm (p=0.031). In other words, vitamin C injection seems effective and safe for reducing PEP if added to rectal indomethacin. In addition, the combination of indomethacin and vitamin C may effectively control pancreatic enzyme levels at 24 hours after ERCP.
The present study is the first to evaluate the effect of a single dose of vitamin C injection plus administration of rectal indomethacin on alleviating PEP. The placebo-controlled trial and use of higher doses of vitamin C were considered trial limitations. Higher doses of vitamin C may effectively decrease the PEP rate. We only measured amylase and lipase levels in the present study. However, regarding the evaluation of inflammatory biomarkers responsible for PEP, it is strongly recommended that biomarkers of the NF-κB pathway and OS (such as malondialdehyde and glutathione peroxidase) in future studies.
Go to : 
Notes
Author Contributions
Conceptualization: AS, MAb; Data curation: RJM, MAs; Formal analysis: MAs, MAb; Funding acquisition: MAb; Investigation: MAb; Methodology: AS, RJM, MAs; Project administration: MAb; Resources: MAb; Software: AS, RJM, SA, MAs, MAb; Supervision: AS, MAb; Validation: MAb; Visualization: MAb; Writing–original draft: SA, MAb; Writing–review and editing: all authors.
Go to : 
REFERENCES
1. Pereira P, Costa-Moreira P, Macedo G. Cholangiopancreatoscopy: expanding the diagnostic indications of endoscopic retrograde cholangiopancreatography. J Gastrointestin Liver Dis. 2020; 29:445–454.
2. Johnson KD, Perisetti A, Tharian B, et al. Endoscopic retrograde cholangiopancreatography-related complications and their management strategies: a "scoping" literature review. Dig Dis Sci. 2020; 65:361–375.
3. Bhatt H. Post-endoscopic retrograde cholangiopancreatography pancreatitis: an updated review of current preventive strategies. Clin Exp Gastroenterol. 2021; 14:27–32.
4. Bozkurt S, Güner A, Kadıoğlu H, et al. The effects of different mechanisms on the development of post-ERCP pancreatitis in an ERCP model in rats. Turk J Gastroenterol. 2013; 24:469–475.
5. Mohammad Alizadeh AH, Abbasinazari M, Hatami B, et al. Comparison of rectal indomethacin, diclofenac, and naproxen for the prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Eur J Gastroenterol Hepatol. 2017; 29:349–354.
6. Abbasinazari M, Mohammad Alizadeh AH, Moshiri K, et al. Does allopurinol prevent post endoscopic retrograde cholangio-pancreatography pancreatitis? A randomized double blind trial. Acta Med Iran. 2011; 49:579–583.
7. Katsinelos P, Kountouras J, Paroutoglou G, et al. Intravenous N-acetylcysteine does not prevent post-ERCP pancreatitis. Gastrointest Endosc. 2005; 62:105–111.
8. Räty S, Sand J, Pulkkinen M, et al. Post-ERCP pancreatitis: reduction by routine antibiotics. J Gastrointest Surg. 2001; 5:339–345. discussion 345.
9. Hatami B, Kashfi SM, Abbasinazari M, et al. Epinephrine in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a preliminary study. Case Rep Gastroenterol. 2018; 12:125–136.
10. Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2020; 52:127–149.
11. Bopanna S, Nayak B, Prakash S, et al. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017; 17:529–533.
12. Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005; 39:671–686.
13. Scott P, Bruce C, Schofield D, et al. Vitamin C status in patients with acute pancreatitis. Br J Surg. 1993; 80:750–754.
14. Siriwardena AK, Mason JM, Balachandra S, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007; 56:1439–1444.
15. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.
16. Cotton PB, Eisen G, Romagnuolo J, et al. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc. 2011; 73:868–874.
17. Armstrong JA, Cash N, Soares PM, et al. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res. 2013; 47:917–933.
18. Thiruvengadam NR, Kochman ML. Emerging therapies to prevent post-ERCP pancreatitis. Curr Gastroenterol Rep. 2020; 22:59.
19. Bonham MJ, Abu-Zidan FM, Simovic MO, et al. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999; 86:1296–1301.
20. Mohseni Salehi Monfared SS, Vahidi H, Abdolghaffari AH, et al. Antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis: a systematic review. World J Gastroenterol. 2009; 15:4481–4490.
21. Gooshe M, Abdolghaffari AH, Nikfar S, et al. Antioxidant therapy in acute, chronic and post-endoscopic retrograde cholangiopancreatography pancreatitis: an updated systematic review and meta-analysis. World J Gastroenterol. 2015; 21:9189–9208.
22. Köseoğlu H, Solakoğlu T, Başaran M, et al. Risk factors for post-ERCP pancreatitis: it depends on the ERCP indication. Acta Gastroenterol Belg. 2020; 83:598–602.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download