Abstract
Tuberculosis is an adverse event in patients with Crohn’s disease receiving anti-tumor necrosis factor (TNF) therapy. However, tuberculosis presenting as a bronchoesophageal fistula (BEF) is rare. We report a case of tuberculosis and BEF in a patient with Crohn’s disease who received anti-TNF therapy. A 33-year-old Korean woman developed fever and cough 2 months after initiation of anti-TNF therapy. And the symptoms persisted for 1 months, so she visited the emergency room. Chest computed tomography was performed upon visiting the emergency room, which showed BEF with aspiration pneumonia. Esophagogastroduodenoscopy with biopsy and endobronchial ultrasound with transbronchial needle aspiration confirmed that the cause of BEF was tuberculosis. Anti-tuberculosis medications were administered, and esophageal stent insertion through endoscopy was performed to manage the BEF. However, the patient’s condition did not improve; therefore, fistulectomy with primary closure was performed. After fistulectomy, the anastomosis site healing was delayed due to severe inflammation, a second esophageal stent and gastrostomy tube were inserted. Nine months after the diagnosis, the fistula disappeared without recurrence, and the esophageal stent and gastrostomy tube were removed.
Anti-tumor necrosis factor (TNF) agents have been effectively used in the treatment of inflammatory bowel disease, including Crohn’s disease (CD); hence, as the prevalence of CD increases in Asian countries, including Korea, so does the use of anti-TNF agents.1-5 However, anti-TNF therapy may cause tuberculosis as an adverse event; therefore, latent tuberculosis screening is recommended before initiation of anti-TNF therapy.6
A case of bronchoesophageal fistula (BEF) caused by tuberculosis has been previously reported7; however, to the best of our knowledge, there have been no reported cases of tuberculosis presenting as BEF in patients with CD receiving anti-TNF therapy. We report a case of tuberculosis presenting as a BEF fistula in a patient with CD who received anti-TNF therapy for 2 months.
A 33-year-old Korean woman was diagnosed with CD 1 year ago and was prescribed mesalazine 3 g/day at the time of diagnosis. Four months prior to the current visit, she was hospitalized for CD exacerbation and received systemic corticosteroid (methylprednisolone) 0.6 mg/kg/day as well as azathioprine 25 mg/day. However, her condition did not improve, so she was started on infliximab (0.5 mg/kg). Latent tuberculosis screening was performed before initiation of infliximab therapy. The tuberculin skin test result was negative (induration 0 mm), the interferon-gamma release assay (IGRA) results were indeterminate, and chest radiography showed no active lung lesion and no evidence of past tuberculosis. After infliximab administration, the patient showed improvement and was discharged. After 6 weeks, she received a third dose of infliximab. Two months after initiation of infliximab therapy, she had fever, cough, and rhinorrhea. These symptoms worsened for 1 month, and yellowish sputum also developed. Thus, she visited to the emergency room.
At the time of the emergency room visit, her blood pressure was 109/81 mmHg, her heart rate was 145 beats per minute, her body temperature was 38.8°C, and her peripheral capillary oxygen saturation level was 100% (room air). She had fever, chills, cough, and yellowish sputum production, with no other symptoms. Physical examination revealed coarse breath sounds with crackles in both lungs. The laboratory findings were as follows: white blood cell count, 20,300/μL (polymorphonuclear neutrophils, 84.2%; lymphocyte, 7.4%); hemoglobin level, 11.0 g/dL; platelet count, 495,000/μL; serum albumin level, 2.7 g/dL; and C-reactive protein level, 20.15 mg/dL. The coagulation profile, liver function test results, and serum creatinine levels were normal. On chest radiography, consolidation was observed in both the middle and lower lung zones. Chest computed tomography (CT) revealed multifocal ill-defined patchy consolidation, ground-glass opacity, peribronchial infiltrates, and ill-defined centrilobular nodules in both the middle and lower lung zones. Additionally, extensive lymph node enlargement was observed in the bilateral mediastinum, which encased the central airway. Luminal narrowing of the left main bronchus was also observed. Moreover, there was a suspicious air tract between the esophagus and bronchus, suggesting a BEF (Fig. 1A, B).
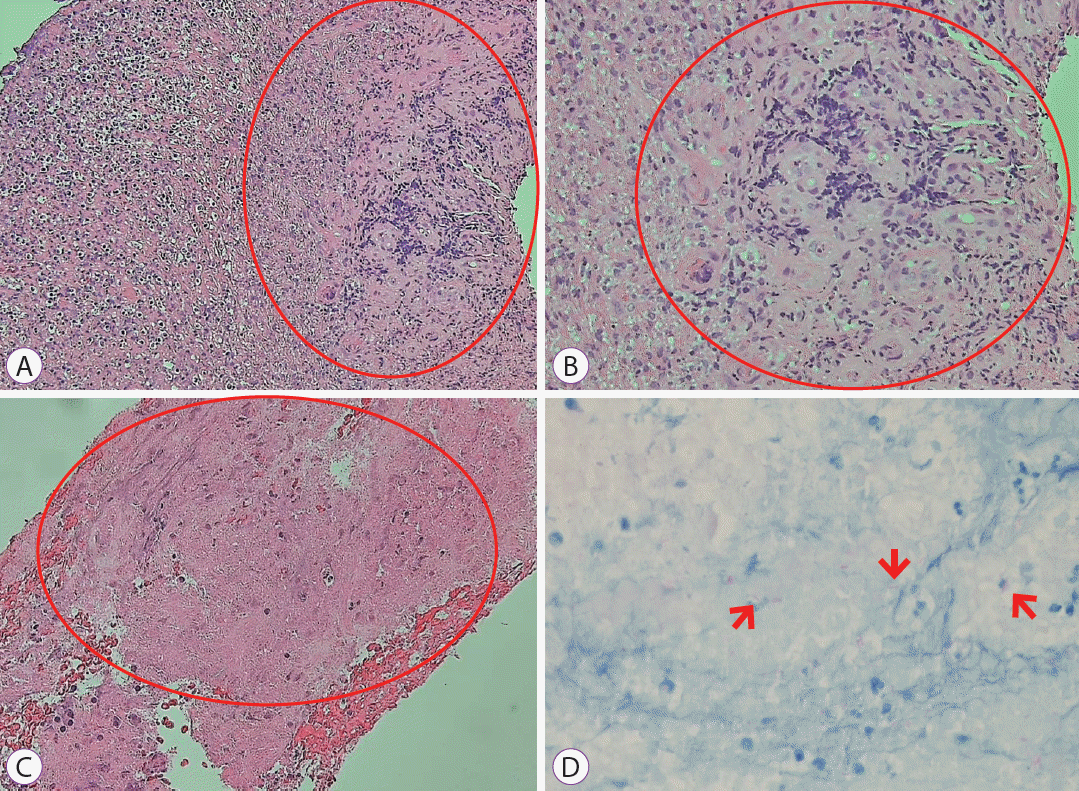
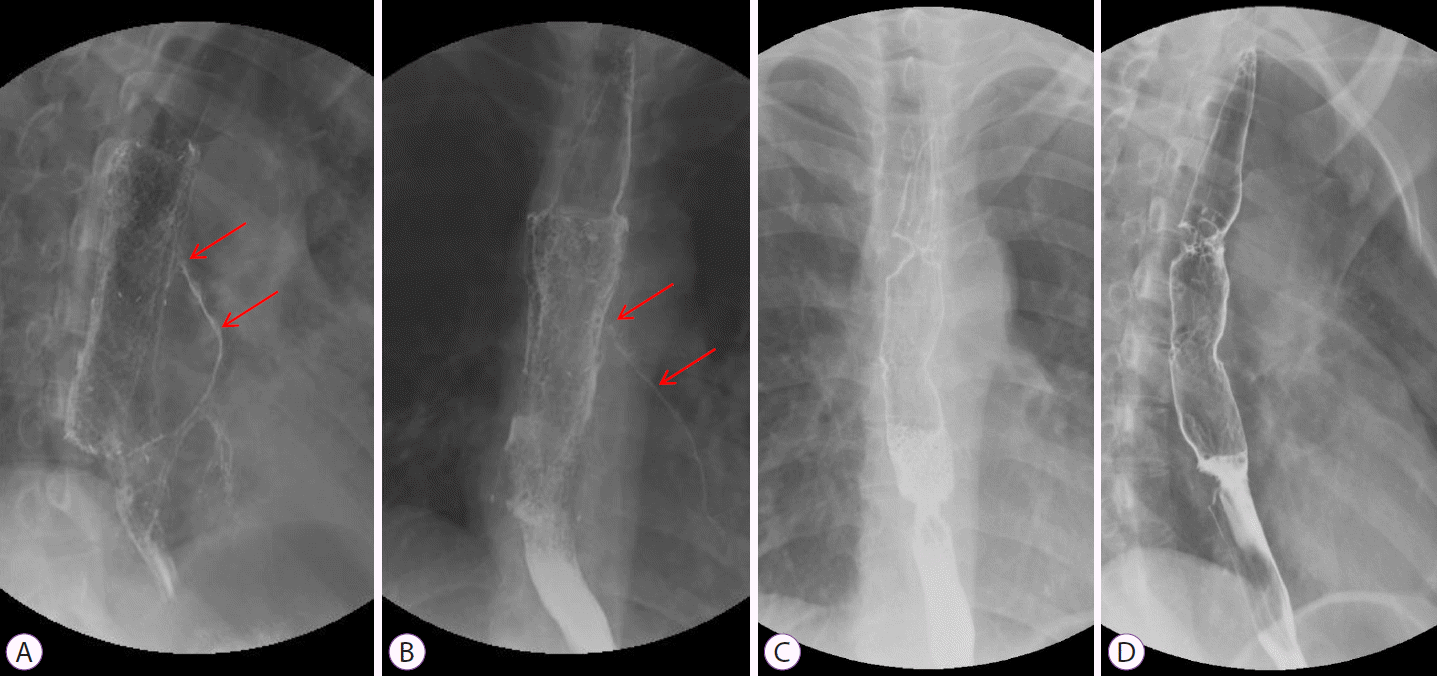
She was admitted to the general ward and received intravenous antibiotics (piperacillin/tazobactam) to manage aspiration pneumonia. Esophagography revealed contrast leakage from the esophagus to the left bronchus, and the presence of a fistula was confirmed (Fig. 1C). Esophagogastroduodenoscopy (EGD) was performed to evaluate the lesion, which was suspected to be a BEF on chest CT. On EGD, an ulcer covered with whitish exudate was observed in the esophagus at 28 to 32 cm from upper incisor, confirming the possibility of BEF, and a biopsy was performed (Fig. 2). Endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA) was also performed to evaluate mediastinal lymph node enlargement, and biopsy was performed at the lymph node stations 4 L and 7. Esophageal biopsy revealed granulomatous inflammation with granulation tissue and marked necrosis (Fig. 3A, B). Moreover, the tuberculosis polymerase chain reaction results were positive. In the EBUS-TBNA specimen, necrotizing granulomatous inflammation was found, which is typical for tuberculosis, and the specimen stained positively for acid-fast bacilli (Fig. 3C, D). Sputum testing was performed, and the Xpert MTB/RIF assay result was positive, along with the acid-fast bacilli stain result. We diagnosed BEF due to tuberculosis as an adverse effect of anti-TNF therapy; therefore, infliximab was discontinued, anti-tuberculosis medications were administered (isoniazid, 300 mg; rifampicin, 300 mg; pyrazinamide, 1,250 mg; and ethambutol, 800 mg), and an esophageal stent was inserted (covered stent, 22 mm×100 mm) through endoscopy (Fig. 4). One week after esophageal stent insertion, the patient showed improvement and was discharged.
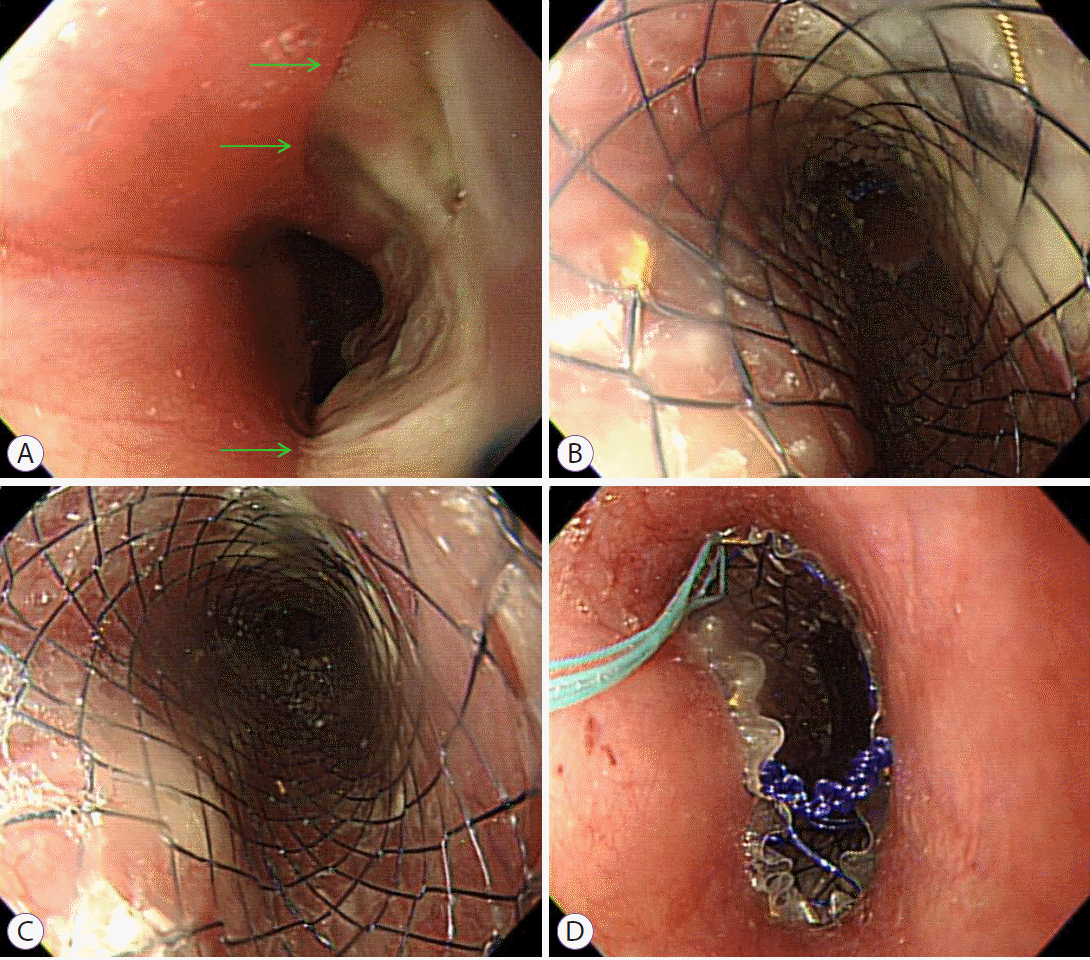
Two months after diagnosis, she developed intermittent aspiration pneumonia again for 1 month. She underwent esophagography and contrast media leakage from the esophagus to the left lower lobar bronchus, where the BEF persisted (Fig. 5A, B). She underwent surgical intervention, and the operative findings revealed a fistula between the esophagus and the left main bronchus. Therefore, fistulectomy was performed. After the operation, the healing of the anastomosis site was delayed due to severe inflammation caused by tuberculosis, resulting in the persistence of the BEF. Moreover, her lung condition was poor due to repeated episodes of aspiration pneumonia, and she had severe esophageal inflammation. Therefore, additional surgical interventions could not be performed. Finally, she underwent insertion of a second esophageal stent and a gastrostomy tube for non-oral enteric nutrition.
Following the operation, second esophageal stent insertion, and gastrostomy tube insertion, the patient showed symptomatic improvement. Anti-tuberculosis treatment was completed 8 months after diagnosis, and chest CT clearly showed resolution of the fistula. Oral feeding was initiated, and no aspiration symptoms were observed. Finally, 9 months after diagnosis, we removed the esophageal stent and gastrostomy tube and performed esophagography to confirm the disappearance of the fistula (Fig. 5C, D). There was no fistula recurrence thereafter.
During treatment for BEF and tuberculosis, CD was maintained in remission with mesalazine 3 g/day alone. Remission was maintained for a short period following the end of the treatment for BEF and tuberculosis; however, the patient experienced CD exacerbation 16 months later. Therefore, she resumed infliximab and was maintained in remission with no recurrence of tuberculosis for 24 months.
BEF is known to occur as a rare complication in malignancies, such as esophageal and lung cancer in adults, as well as in infectious conditions, such as tuberculosis.7,8 Congenital BEF is mostly found in childhood and is very rare in adults.9 There have been reports of BEF due to esophageal tuberculosis,10 and recently, BEF has been reported in patient with coinfection of severe acute respiratory syndrome coronavirus 2 and tuberculosis.11 In patients with CD, there have been some cases of BEF due to esophageal involvement,12 although esophageal involve ment in CD is rare and is reportedly difficult to diagnose.13 There have thus far been two cases that reported BEF with congenital causes.14,15
Tuberculosis may occur as an adverse effect of anti-TNF therapy in patients with CD. To prevent tuberculosis, tuberculin skin test and IGRA are performed to screen for latent tuberculosis before anti-TNF therapy initiation.6 However, it has been reported that tuberculosis may occur even if both test results are negative.16 In addition, the results of both tests may not match, especially when an immunomodulator is used, which negatively affects the results of the IGRA.17 Similarly, in our case, the latent tuberculosis screening results were negative before anti-TNF therapy was initiated, but tuberculosis occurred later on. The immunomodulator may have resulted in false-negative results on the screening test.
In general, BEFs due to nonmalignant causes have been treated surgically. Surgical treatment has shown good long-term outcomes, and the rate of definitive fistula closure is reported to be up to 90% to 95%.18 Endoscopic treatment has been attempted to manage inoperable malignant BEF or in cases that call for less invasive methods. According to the European Society of Gastrointestinal Endoscopy guidelines, an esophageal self-expendable metal stent can be applied to a malignant BEF, with a clinical success rate of 56% to 100%.19
In our case, BEF and aspiration pneumonia occurred in a patient with CD who received anti-TNF therapy. Furthermore, tuberculosis was confirmed to be the cause of BEF through EGD with biopsy and EBUS-TBNA. The patient stopped anti-TNF therapy and started anti-tuberculosis medication. She underwent fistulectomy with primary closure due to a lack of improvement following esophageal stenting. Our case suggests that if a patient with CD on anti-TNF therapy has symptoms such as fever, cough, and sputum production, it is necessary to examine the possibility of tuberculosis and its complications.
REFERENCES
1. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019; 13:1410–1417.
2. Kaibullayeva J, Ualiyeva A, Oshibayeva A, et al. Prevalence and patient awareness of inflammatory bowel disease in Kazakhstan: a cross-sectional study. Intest Res. 2020; 18:430–437.
3. Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest Res. 2019; 17:54–62.
4. Wilkens R, Novak KL, Maaser C, et al. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: current status and future perspectives. Therap Adv Gastroenterol. 2021; 14:17562848211006672.
5. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res. 2020; 18:345–346.
6. Park DI, Hisamatsu T, Chen M, et al. Asian organization for Crohn’s and colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.
7. Spalding AR, Burney DP, Richie RE. Acquired benign bronchoesophageal fistulas in the adult. Ann Thorac Surg. 1979; 28:378–383.
8. Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008; 34:1103–1107.
9. Holder TM, Cloud DT, Lewis JE, et al. Esophageal atresia and tracheoesophageal fistula. A survey of its members by the surgical section of the american academy of pediatrics. Pediatrics. 1964; 34:542–549.
10. Desai P, Mayenkar P, Northrup TF, et al. Bronchoesophageal fistula due to esophageal tuberculosis. Case Rep Infect Dis. 2019; 2019:6537437.
11. Aissaoui H, Louvel D, Drak Alsibai K. SARS-CoV-2 and mycobacterium tuberculosis coinfection: a case of unusual bronchoesophageal fistula. J Clin Tuberc Other Mycobact Dis. 2021; 24:100247.
12. Clarke BW, Cassara JE, Morgan DR. Crohn’s disease of the esophagus with esophagobronchial fistula formation: a case report and review of the literature. Gastrointest Endosc. 2010; 71:207–209.
13. Saadah OI, Fallatah KB, Baumann C, et al. Histologically confirmed upper gastrointestinal Crohn’s disease: is it rare or are we just not searching hard enough? Intest Res. 2020; 18:210–218.
14. Devbhandari MP, Raco L, Hendrickse MT, et al. Congenital bronchoesophageal fistula in a patient with Crohn’s disease: a cautionary tale. Ann Thorac Surg. 2005; 79:1776–1777.
15. Kato H, Yoshikawa M, Saito T, et al. Congenital bronchoesophageal fistula with Crohn’s disease in an adult: report of a case. Surg Today. 2001; 31:446–449.
16. Agarwal A, Kedia S, Jain S, et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest Res. 2018; 16:588–598.
17. Alrajhi S, Germain P, Martel M, et al. Concordance between tuberculin skin test and interferon-gamma release assay for latent tuberculosis screening in inflammatory bowel disease. Intest Res. 2020; 18:306–314.
18. Bibas BJ, Cardoso PFG, Minamoto H, et al. Surgery for intrathoracic tracheoesophageal and bronchoesophageal fistula. Ann Transl Med. 2018; 6:210.
19. Spaander MCW, van der Bogt RD, Baron TH, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) guideline-update 2021. Endoscopy. 2021; 53:751–762.
Fig. 1.
(A, B) Chest computed tomography shows an air cleft between the esophagus and the left main bronchus (in the red circles), suggesting bronchoesophageal fistula and aspiration pneumonia. (C) Esophagography shows contrast media leakage from the esophagus to the left bronchus (red arrows).
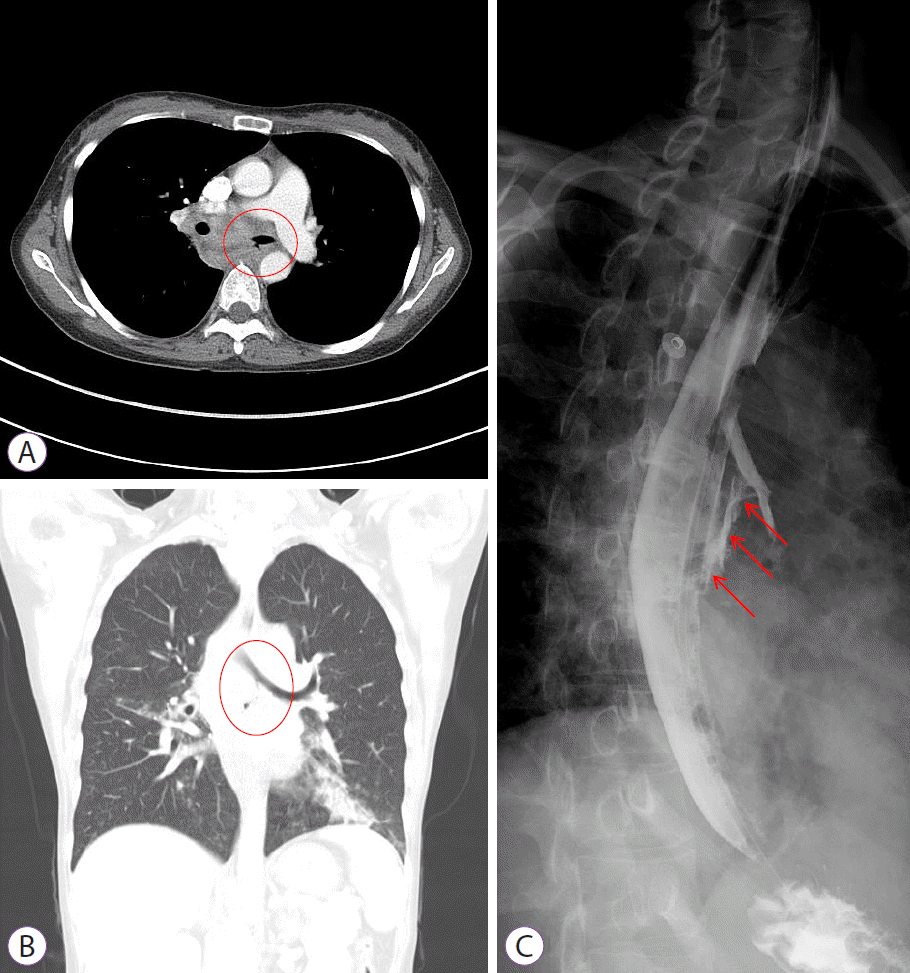
Fig. 2.
(A, B) Esophagogastroduodenoscopy shows a deep ulcer (in the green circles) in the esophagus at 28 to 32 cm from upper incisor.
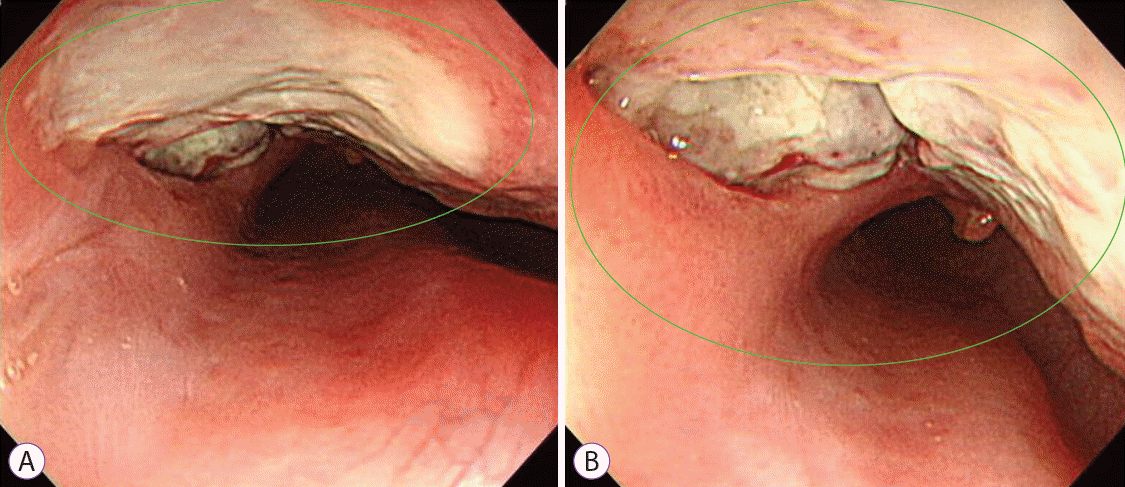
Fig. 3.
(A, B) Esophagogatroduodenoscopic biopsy shows granulomatous inflammation with granulation tissue and marked necrosis (in the red circles). Hematoxylin and eosin stain: (A) ×100, (B) ×200. (C, D) Endobronchial ultrasound with transbronchial needle aspiration shows chronic granulomatous inflammation with necrosis (in the red circle), and many acid-fast positive bacilli (red arrows) are identified by acid-fast bacilli staining. (C) Hematoxylin and eosin stain ×200. (D) Acid-fest bacilli stain ×400.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download