Ethical statements: This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 2023-0013). The need for written informed consent from the participants was waived because of the retrospective nature of this study.
A 65-year-old male visited the emergency room with abdominal pain on the right upper quadrant. His past medical history included cholecystectomy for cholecystitis 39 years ago. His physical examination results were as follows: blood pressure, 110/70 mmHg; pulse rate, 78 beats/min; respiratory rate, 20 breaths/min; and body temperature, 36.6 °C. There was no tenderness on the right upper quadrant abdomen and Murphy sign was negative. His laboratory tests included the following: white blood cells, 7,530/μL; hemoglobin, 11.7 g/dL; platelet, 158,000/μL; total bilirubin, 0.5 mg/dL; aspartate aminotransferase, 346 IU/L; alanine aminotransferase, 255 IU/L; alkaline phosphatase, 298 IU/L; gamma-glutamyl transferase, 604 IU/L; amylase, 40 IU/L; lipase, 7 U/L; C-reactive protein, 14.41 mg/dL; carcinoembryonic antigen (CEA), 4.44 ng/mL; and carbohydrate antigen 19-9, 25.3 U/mL. Viral markers for hepatitis A, B, and C were all negative, and blood culture showed no bacterial growth. Abdominal computed tomography revealed an 8 mm polypoid lesion at the right posterior hepatic duct (RPHD); intraductal papillary neoplasm of the bile duct was suspected (
Fig. 1). On suspicion of cholangitis, we started empirical antibiotic therapy; subsequently, the symptoms and laboratory findings improved. In magnetic resonance cholangiopancreatography (MRCP), a triangular-shaped polypoid lesion at the RPHD was detected, suggesting early cholangiocarcinoma (
Fig. 2). MRCP also revealed an anomalous variation of the RPHD directly draining into the common hepatic duct (
Fig. 2). Positron emission tomography showed no evidence of a hypermetabolic lesion. Therefore, we performed endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound for the differential diagnosis of the RPHD lesion. ERCP revealed a focal filling defect with a smooth margin of the RPHD. In endoscopic ultrasound, a 7.8 mm hypoechoic lesion at the RPHD was noted, but infiltration into the hepatic duct wall was not clearly seen (
Fig. 3). We decided a short-term follow-up because we could not rule out benign diseases such as fibrous change of the RPHD resulting from the previous cholecystectomy. One and a half month later, abdominal computed tomography identified an interval increase in size of the polypoid lesion at the RPHD, with strong contrast enhancement (
Fig. 4). Laboratory test included the following: aspartate aminotransferase, 17 IU/L; alanine aminotransferase, 29 IU/L; alkaline phosphatase, 62 IU/L; C-reactive protein, 0.54 mg/dL; CEA, 7.78 ng/mL; and carbohydrate antigen 19-9, 16.6 U/mL, and only CEA was increased. In ERCP, the filling defect of the RPHD became more prominent than that in the previous ERCP (
Fig. 5). Hence, biopsy through ERCP was performed, but the pathologic result showed only a few benign biliary epithelial strips. Eventually, we decided to perform a surgery, considering that cholangiocarcinoma could not be excluded because of the progress of the lesion and the repeated right upper quadrant pain during the follow-up period. Intraoperatively, the palpable mass was found at the RPHD. The common hepatic duct and CBD remained patent. Therefore, we performed right posterior sectionectomy of the liver with a negative surgical margin of the RPHD (
Fig. 6). The patient’s postoperative course was uneventful, and he was discharged without any complication. Surgical pathology revealed TN, with no evidence of malignancy. Microscopically, an oval-shaped nodule was observed under the biliary epithelium. The nodule consisted of haphazardly arranged spindle cells with no nuclear atypia. Immunohistochemically, the spindle cells positively reacted to S100 protein (
Fig. 6).
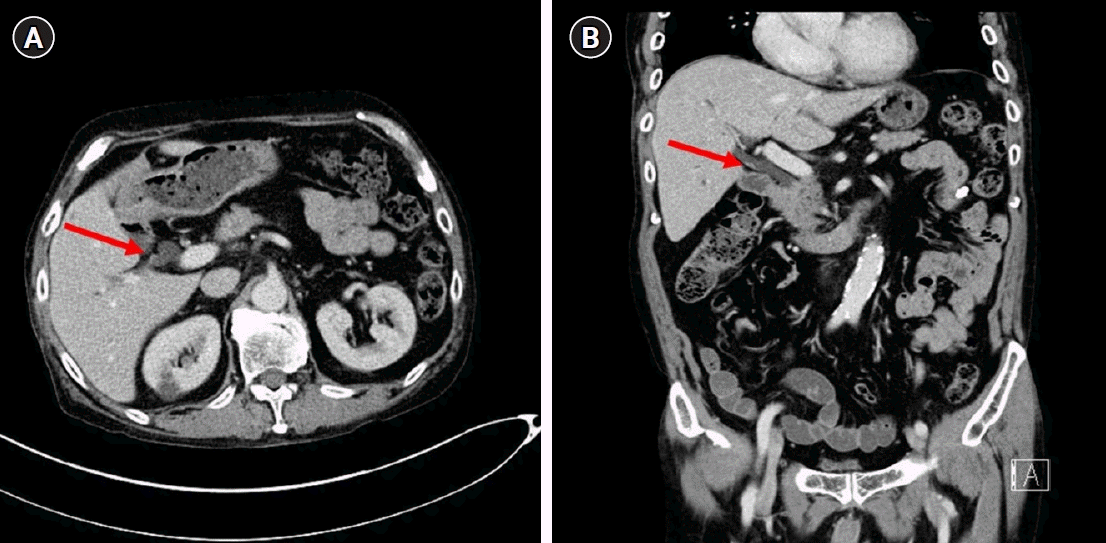 | Fig. 1.An 8-mm polypoid lesion at the right posterior hepatic duct; (arrow) an intraductal papillary neoplasm of the bile duct was suspected. (A) Axial view. (B) Coronal view. 
|
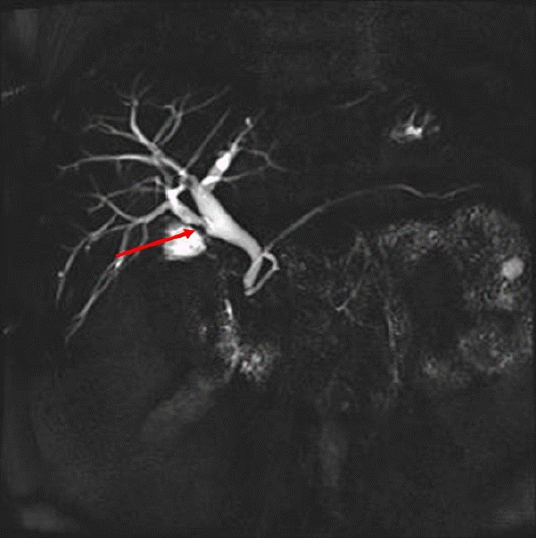 | Fig. 2.A triangular-shaped polypoid lesion at the right posterior hepatic duct was detected (arrow), suggesting early cholangiocarcinoma. 
|
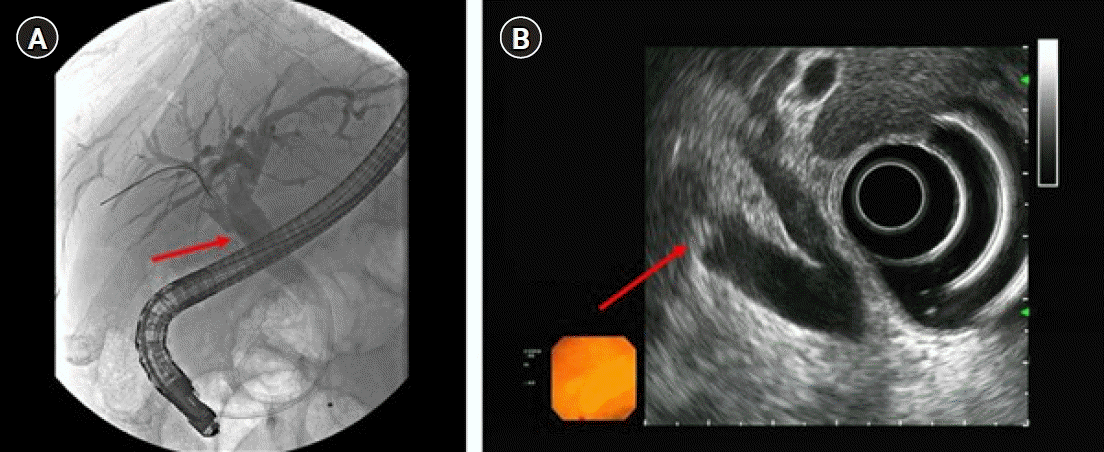 | Fig. 3.A focal filling defect with a smooth margin at the right posterior hepatic duct (arrow). (A) Endoscopic retrograde cholangiopancreatography and (B) endoscopic ultrasonography. 
|
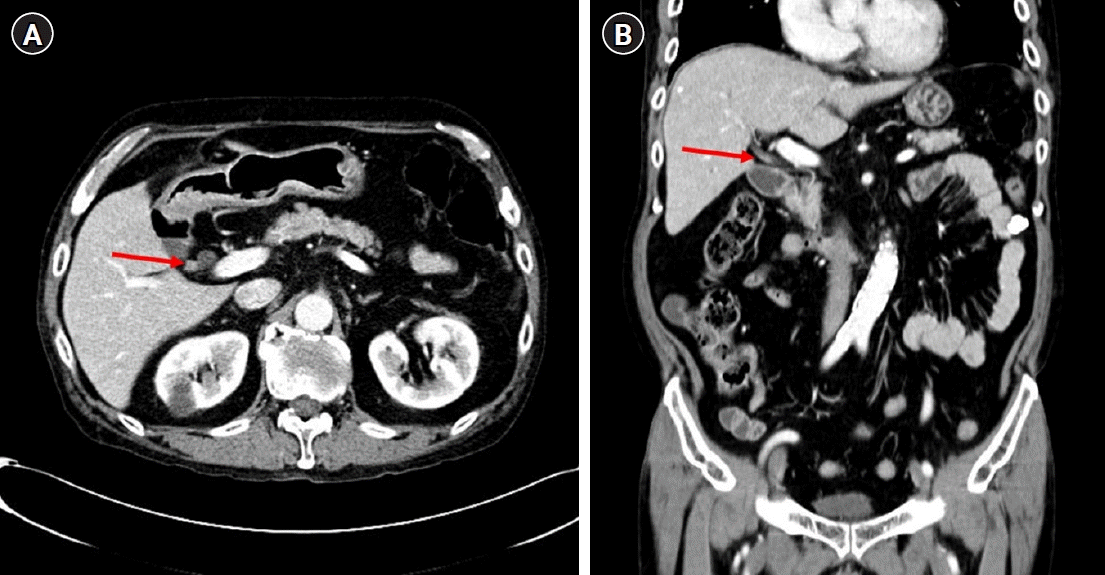 | Fig. 4.An interval increase in the size of the polypoid lesion at the right posterior hepatic duct, with strong contrast enhancement (arrow). (A) Axial view. (B) Coronal view. 
|
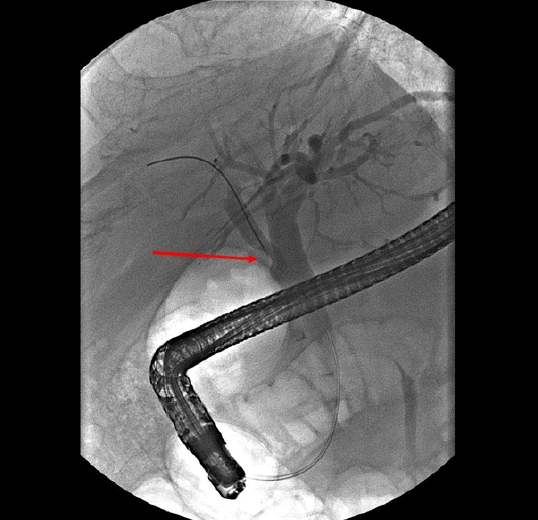 | Fig. 5.The filling defect of the right posterior hepatic duct became more prominent than that in the previous endoscopic retrograde cholangiopancreatography (arrow). 
|
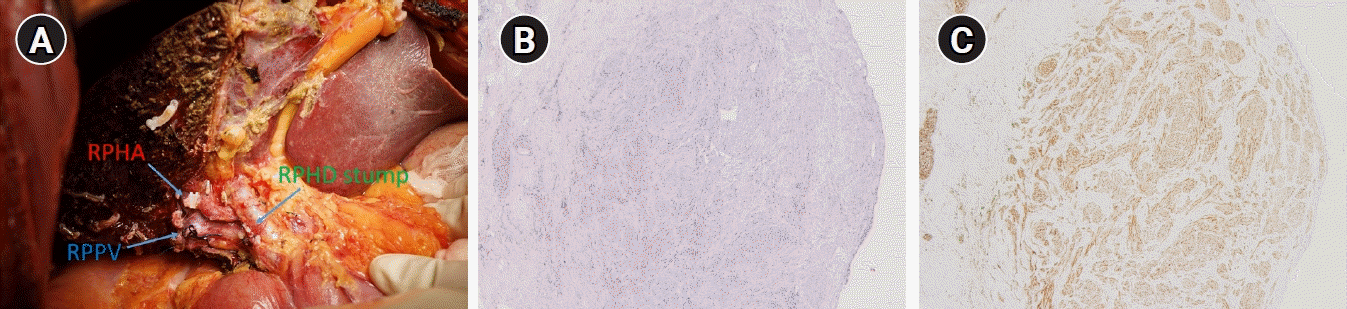 | Fig. 6.(A) Right posterior sectionectomy of the liver with a negative surgical margin of the right posterior hepatic duct (RPHD). RPHA, right posterior hepatic artery; RPPV; right posterior portal vein. Oval-shaped nodule, consisting of haphazardly arranged spindle cells with no nuclear atypia. Immunohistochemically, the spindle cells positively reacted to S100 protein (B: hematoxylin and eosin stain, ×40, C: ×40). 
|










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download