Abstract
Background
Benign bladder tumors are rare disease entities, and insufficient studies have assessed their epidemiological characteristics. The authors investigated the prevalence of benign bladder tumors by retrospectively investigating pathology reports of transurethral resection of bladder tumor (TURBT) procedures over the past 20 years.
Methods
We analyzed 1,674 pathology reports of TURBT conducted in 1,160 patients from January 1, 2000, to April 30, 2022. The prevalence of benign tumors and histological classification according to the presence of primary (group 1) and recurrent (group 2) bladder lesions were retrospectively investigated.
Results
The mean age of patients was 65.2±11.5 years, and 1,284 cases (79.1%) were in men. Benign bladder tumors comprised 278 cases (248 patients) accounting for about 17.1% of the total TURBT cases (278/1,624). Furthermore, 184 patients (16.0%, 184/1,147) belonged to group 1 and 78 patients (27.4%, 78/285) belonged to group 2. Among all benign lesions that underwent TURBT, cystitis was the most common (41.0%, 114/278), and this rate was higher in group 2 (64/184 [34.8%] vs. 50/94 [53.2%], p<0.001). The prevalence of non-neoplastic lesions was higher in group 1 (44/184 [23.9] vs. 11/94 [11.7%], p<0.001). There was no difference in the prevalence of noninvasive urothelial neoplasms between the two groups (22/184 [12.0%] vs. 8/94 [8.5%], p=0.86).
Benign bladder tumors are a rare disease entity [1,2]. Their types are diverse ranging from non-neoplastic lesions, such as Brunn nest and inverted papilloma, to inflammatory lesions, such as cystitis cystica and cystitis glandularis [3,4]. In some cases, a tumor is found to be benign after transurethral resection of bladder tumor (TURBT) is performed to remove tumors in relation to a papillary tumor or a granular lesion found on cystoscopy; in other cases, a tumor is found to be benign on an active biopsy performed when the tumor is thought to be a recurrent malignant tumor or an ambiguous lesion is found during regular follow-up with cystoscopy after bladder cancer surgery. As there is inadequate data on their incidence and frequent histological types, urologists actively perform TURBT for tissue confirmation. However, aggressive TURBT for all lesions suspected of recurrent bladder cancer may lead to over-treatment. Recently, the feasibility of active surveillance has increased due to the postponement of elective surgery and restricted access to hospitals caused by the coronavirus disease 2019 (COVID-19) pandemic [5]. In a study of the Bladder Cancer Italian Active Surveillance (BIAS) group, about one-third cases of lesions were diagnosed as benign lesions after TURBT, which progressed during active surveillance of low-grade-like bladder tumor, it could be seen that, in the case of low-risk appearance bladder lesion, the frequency of benign tumors is not low [6]. Therefore, the authors investigated the frequency and histological types of benign tumors by retrospectively comparing primary and recurrent benign bladder tumors diagnosed during the follow-up of primary benign bladder tumors and bladder cancers.
Ethical statements: This study was approved by the Institutional Review Board of the Kosin University Gospel Hospital (IRB No. KUGH 2022-08-030). The informed consent was waived because this design is a retrospective study.
Among a total of 1,624 cases in which 1,160 patients underwent TURBT from January 2000 to April 2022, the medical records of 1,624 cases and 1,140 patients were retrospectively investigated, after excluding 50 cases in which a second TURBT was performed for residual tumors in patients found to have a malignant tumor and repeat TURBT was performed for the purpose of identifying residual tumors after TURBT in another hospital. The basic principle of TURBT was removal of all possible visible lesions including the muscle layer. In the case of non-muscle invasive bladder cancer, cystoscopy was conducted at an interval of 3 months for 2 years and at an interval of 6 months for 5 years after TURBT, and in the case of benign bladder cancer, although there was no follow-up consensus, cystoscopy was conducted once every 6 months for 2 years [7]. Benign bladder tumors were classified into a total of five groups; non-neoplastic lesions, cystitis, noninvasive urothelial neoplasm, mesenchymal and other tumors, and others based on the World Health Organization (WHO) 2004/2016 classification system [8]. Among them, the cystitis group was classified as a separate group since it includes all types of cystitis, such as polypoid-papillary cystitis and follicular cystitis, and it accounts for a considerable number among the total specimens, although it is not included in urothelial neoplasia. The patient groups were classified into primary bladder tumors (group 1) and recurrent benign bladder tumors found during regular follow-up after TURBT for a malignant or benign tumor (group 2), and the histological characteristics of the two groups were compared using the Student t-test. The data were analyzed using SPSS version 27.0 (IBM Corp.). If p<0.05, it was considered statistically significant.
The mean age of all patients was 65.2±11.5 years, and males accounted for 1,284 cases (79.1%). The number of cases diagnosed with a benign bladder tumor was 278 (248 patients), accounting for about 17.1% of all TURBT cases. Among the 278 cases diagnosed with a benign bladder tumor, 184 patients (16.0%, 184/1,147) belonged to group 1 and 78 patients (27.4%, 78/285) belonged to group 2, showing that the percentage of benign bladder tumors was higher in group 2 (p=0.021). The most common pathological outcome was cystitis (41.0%, 114/278). The total number of cases of papillary urothelial neoplasm of low malignant potential (PUNLMP) were 22 in group 1 and eight in group 2, accounting for 12.0% and 8.5%, respectively. PUNLMP was excluded from the incidence of benign tumors since these lesions fall under a borderline by a pathophysiologic taxonomy [8]. Non-neoplastic lesions were detected in 55 cases, noninvasive urothelial neoplasms in 30 cases, mesenchymal and other tissue lesions in nine cases, and others in 70 cases. A total of 55 cases of non-neoplastic lesions comprised 14 cases of Brunn nest (25.5%), followed by nine cases of cystitis glandularis (16.4%), eight cases of squamous metaplasia (14.5%), and seven cases of urothelial hyperplasia (12.7%). A total of 30 cases of noninvasive urothelial neoplasms comprised 15 cases of inverted papilloma (50.0%), followed by 10 cases of urothelial dysplasia (33.3%) and five cases of urothelial papilloma (16.7%). In the group of others, most tissues were not urothelial tissues, such as prostate tissues, necrotic tissues, and inflammatory tissue, or they could not be classified into the above categories. Except the groups of cystitis and others, the most common form of benign bladder tumor was inverted papilloma (14 cases, 18.7%) in group 1 and Brunn nest (5 cases, 26.3%) in group 2. With respect to the histological characteristics of group 1 and group 2, the incidence of cystitis was higher in group 2 (64/184 [34.8%] vs. 50/94 [53.2%], p<0.001) and the incidence of non-neoplastic lesion was higher in group 1 (44/184 [23.9%] vs. 11/94 [11.7%], p<0.001). In addition, there was no difference in the incidence of noninvasive urothelial neoplasm between the two groups (22/184 [12.0%] vs. 8/94 [8.5%], p=0.86) (Table 1). Among the 285 patients who underwent TURBT on two or more occasions, the number of patients who were diagnosed with a benign bladder tumor on both TURBT procedures was four, the number of patients who were diagnosed with a benign bladder tumor on the first TURBT but were diagnosed with a malignant tumor on surgery later was 16, and the number of patients who were diagnosed with bladder cancer on the first TURBT but were diagnosed with a benign bladder tumor later on surgery was 55.
Various types of benign bladder tumors are already known, such as inverted papilloma, Brunn nest, and urothelial papilloma (Fig. 1) [8]. Many reports, such as case reports, focusing on pathological outcomes have documented each histological type in various ways (Fig. 2). However, there is a lack of adequate data on the prevalence of benign tumors found in all bladder tumors. In the present study, the frequency of benign bladder tumors after TURBT was 17.1%, and the incidence was higher in suspicious recurrent lesions than in primary bladder tumors by almost 2-fold (27.4% vs. 16.0%). The most common among all benign tumors in the present study was the cystitis group, which included polypoid-papillary cystitis and follicular cystitis. It may be difficult to distinguish such lesions from many papillary urothelial neoplasms and they account for a large proportion of cases in which TURBT is performed and these lesions mimic a bladder tumor. If what a urologist has found on cystoscopy is close to an inflammatory lesion, pathologists should hesitate to diagnose the lesion as urothelial neoplasia [9]. Although it is difficult to histopathologically differentiate PUNLMP, urothelial dysplasia, and urothelial papilloma from each other, since their recurrence and progression are different from each other, it is important to distinguish these lesions while diagnosing them [10,11]. In the present study, the rate of PUNLMP was reported to be 12.0% in group 1 and 8.5% in group 2. In the present study, PUNLMP was excluded from the investigation of the frequency of benign tumors since PUNLMP has the characteristics of a borderline tumor in light of the fact that Bobjer et al. [12] have reported that PUNLMP has a recurrence rate of 21%, Maxwell et al. [13] have also once reported a long-term recurrence rate of 20%, and Jones and Cheng [14,15] have argued that PUNLMP should be reclassified as low-grade carcinoma because its recurrence rate is high although its progression to invasive carcinoma is extremely rare. When a benign bladder tumor has an aspect of a papillary mass, it can be identified in an imaging study, such as ultrasonography or computed tomography, in which case TURBT is performed after checking the mass on cystoscopy. However, in the case of no visible mass in an imaging study, the cystoscopic finding is usually flat, edematous and inflammatory lesion. Thus, the cystoscopic finding is important to decide whether to perform TURBT or not. It can also depend on the urologist’s experience. The more the TURBT is performed actively, the further the incidence of benign lesions, such as cystitis, may increase, which may lead to unnecessary TURBT procedures causing an increase in the overall medical costs. In the present study, the percentage of cystitis was higher in recurrent lesions than in primary bladder tumors (34.8% vs. 53.2%). It will be possible to reduce unnecessary TURBT procedures when the indications for TURBT are well established in the lesions suspected of showing recurrence. In a study by Hernandez et al. [16], it was decided to perform interventions, such as TURBT, during active surveillance of low-grade bladder carcinoma, if there were tumor-related symptoms, hematuria, progressive suspicious lesions, malignant cells on urine cytology, or an increased number of tumors. With respect to the lesions suspected of showing recurrence, which are found during the follow-up of bladder carcinoma, since cystoscopic findings of recurrence can show discrepancy between urologists and may depend on the experience of the urologist, each urologist may have a different opinion on whether or not it is a case of suspected recurrence. Traditionally, bacillus Calmette-Guerin instillation is known to cause granuloma, but as the surgeon’s experience increases, it is not analyzed separately because it can be sufficiently distinguished cystoscopically. In the case of a urologist with accumulated experience, active surveillance of suspected lesions may reduce unnecessary TURBT procedures. In addition, if office fulguration is possible in an out-patient clinic, as reported by Soloway [17], it can reduce cumbersome anesthesia, hospitalization, and surgery; in which case, since TURBT for suspected lesions during follow-up can decrease, the frequency of benign bladder tumors that recur during follow-up can also be reduced. The authors have reported that the frequency of benign bladder tumors has increased after the introduction of a high-definition flexible cystoscopy system using narrow-band images. We believe that benign lesions were increased temporarily as suspected lesions could be found more easily because higher resolution images could be viewed using the technique [18]. However, as our experience increased, we have tried to reduce unnecessary TURBT procedures by active surveillance if there is no change cystoscopically. In the present study, the data of three experienced surgeons were utilized and there was a limitation as standardization was not achieved for each surgeon since there was no agreement on the opinions on a recurrent lesion. However, in the study by the BIAS study group, they reported similar results; a considerable percentage of 32.8% in the case of a short-term follow-up and that 19.2% in the case of a long-term follow-up [6,19]. These results did not show many differences from those in the present study.
In conclusion, the probability of a benign lesion during TURBT surgery was 17.1%, among which cystitis was the most common. In the case of TURBT for recurrent lesions, the frequency of benign tumors was higher than that of primary benign bladder tumors, and the frequency of cystitis was significantly higher than that of primary benign tumors. In primary benign bladder tumors, although the frequency of non-neoplastic lesions was higher, there was no difference in the incidence of noninvasive urothelial neoplasm between the two groups.
Notes
Author contributions
Conceptualization: TK, SK. Data curation: JB, YS, DK. Formal analysis: WS, SK. Investigation: JB, YS, DK. Methodology: SK, TK. Project administration: TK. Resources: JB, BC. Software: TK. Supervision: TK. Validation: TK. Visualization: BC. Writing - original draft: JB. Writing - review & editing: TK.
References
1. Mendes JE, Ferreira AV, Coelho SA, Gil C. Bladder leiomyoma. Urol Ann. 2017; 9:275–7.
2. Monappa V, Jaiprakash P, Thomas J, Hegde P. Bladder paraganglioma: a report of two cases. Afr J Urol. 2018; 24:70–2.
3. Picozzi S, Casellato S, Bozzini G, Ratti D, Macchi A, Rubino B, et al. Inverted papilloma of the bladder: a review and an analysis of the recent literature of 365 patients. Urol Oncol. 2013; 31:1584–90.
4. Sweeney MK, Rais-Bahrami S, Gordetsky J. Inverted urothelial papilloma: a review of diagnostic pitfalls and clinical management. Can Urol Assoc J. 2017; 11:66–9.
5. Paciotti M, Contieri R, Fasulo V, Casale P, Saita A, Buffi NM, et al. Active surveillance for recurrent non-muscle invasive bladder cancer: which lessons have we learned during COVID-19 pandemic? Minerva Urol Nephrol. 2022; 74:1–4.
6. Hurle R, Colombo P, Lazzeri M, Lughezzani G, Buffi NM, Saita A, et al. Pathological outcomes for patients who failed to remain under active surveillance for low-risk non-muscle-invasive bladder cancer: update and results from the Bladder Cancer Italian Active Surveillance Project. Eur Urol Oncol. 2018; 1:437–42.
7. Ribeiro A, Pereira M, Reis A, Ferreira G. Urothelial papilloma: a rare cause of gross haematuria in childhood. BMJ Case Rep. 2017; 2017:bcr2017219341.
8. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs: Part B: prostate and bladder tumours. Eur Urol. 2016; 70:106–19.
9. Lane Z, Epstein JI. Polypoid/papillary cystitis: a series of 41 cases misdiagnosed as papillary urothelial neoplasia. Am J Surg Pathol. 2008; 32:758–64.
10. Hodges KB, Lopez-Beltran A, Davidson DD, Montironi R, Cheng L. Urothelial dysplasia and other flat lesions of the urinary bladder: clinicopathologic and molecular features. Hum Pathol. 2010; 41:155–62.
11. Abdel Gawad AM, Rabie A, Abdelwahed MS, Hasan A. Urothelial papilloma of the urinary bladder: a case report and literature review of a rare entity. Cureus. 2022; 14:e22046.
12. Bobjer J, Hagberg O, Aljabery F, Gardmark T, Jahnson S, Jerlstrom T, et al. Bladder cancer recurrence in papillary urothelial neoplasm of low malignant potential (PUNLMP) compared to G1 WHO 1999: a population-based study. Scand J Urol. 2022; 56:14–8.
13. Maxwell JP, Wang C, Wiebe N, Yilmaz A, Trpkov K. Long-term outcome of primary papillary urothelial neoplasm of low malignant potential (PUNLMP) including PUNLMP with inverted growth. Diagn Pathol. 2015; 10:3.
14. Jones TD, Cheng L. Reappraisal of the papillary urothelial neoplasm of low malignant potential (PUNLMP). Histopathology. 2020; 77:525–35.
15. Jones TD, Cheng L. Noninvasive papillary urothelial neoplasia (NIPUN): renaming cancer. Urol Oncol. 2021; 39:286–90.
16. Hernandez V, Llorente C, de la Pena E, Perez-Fernandez E, Guijarro A, Sola I. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol. 2016; 34:165.e19-23.
17. Soloway MS. Active surveillance or office fulguration for low grade ta bladder tumors: a win-win for patients and urologists. J Urol. 2018; 199:1120–2.
18. Seo WT, Kang SH. Postoperative recurrent bladder tumors detection by narrow-band imaging cystoscopy. Korean J Urol Oncol. 2020; 18:209–14.
19. Contieri R, Paciotti M, Lughezzani G, Buffi NM, Frego N, Diana P, et al. Long-term follow-up and factors associated with active surveillance failure for patients with non-muscle-invasive bladder cancer: the Bladder Cancer Italian Active Surveillance (BIAS) experience. Eur Urol Oncol. 2022; 5:251–5.
Fig. 1.
Various cystoscopic findings of benign bladder tumors. (A) Brunn nest, (B) cystitis glandularis, (C) eosinophilic cystitis, (D) inverted papilloma, (E) urothelial dysplasia, and (E) urothelial hyperplasia.
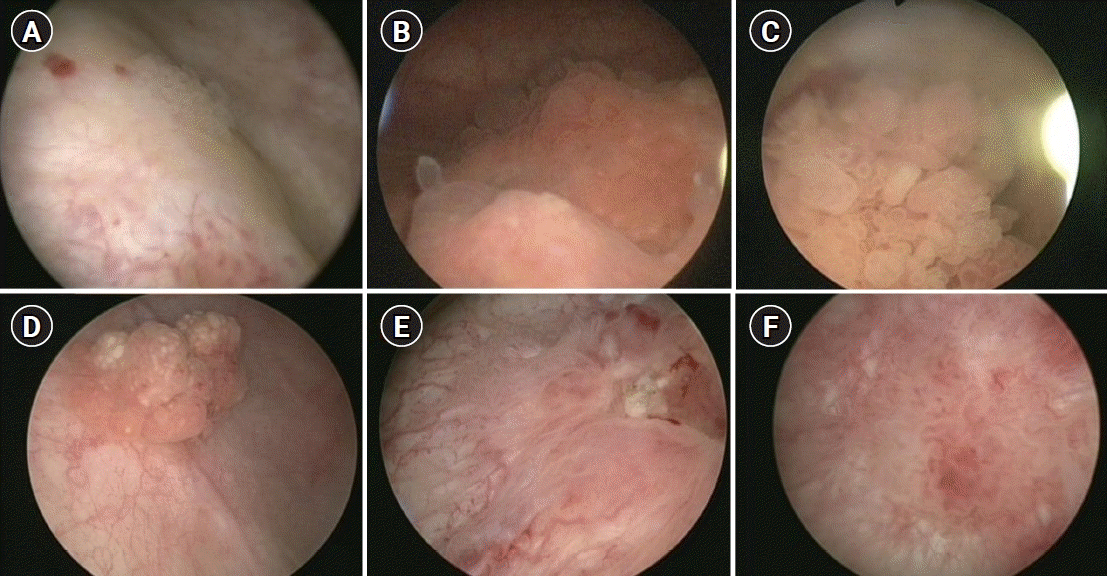
Fig. 2.
Various pathologic findings of benign bladder tumors (hematoxylin and eosin stain). (A) Brunn nest (×200), (B) cystitis glandularis (×100), (C) eosinophilic cystitis (×200), (D) inverted papilloma (×100), (E) urothelial dysplasia (×400), and (E) urothelial hyperplasia (×200).
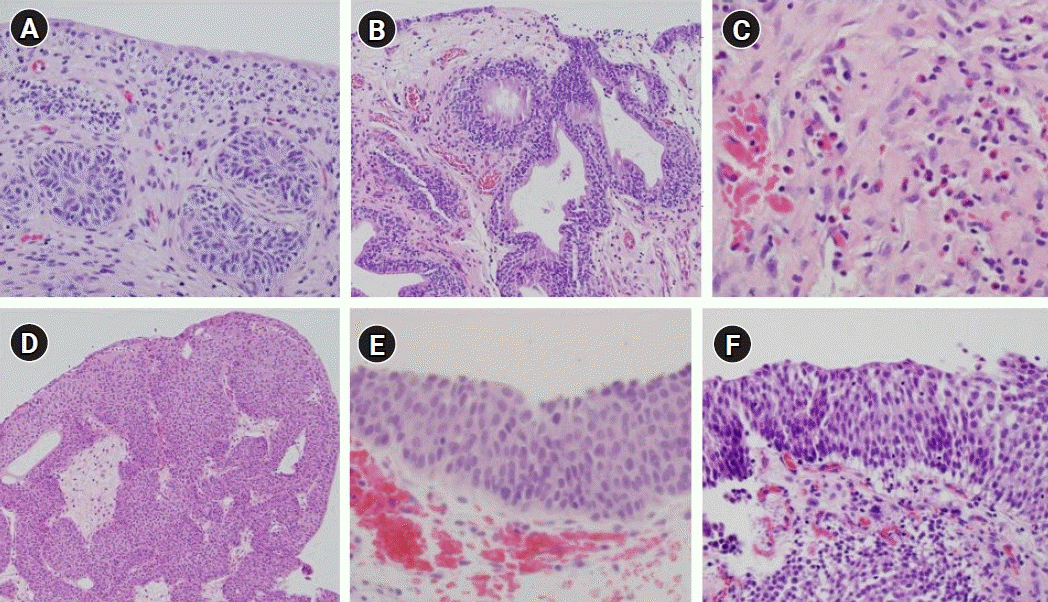
Table 1.
Prevalence of histological types in each group
| Variable | Group 1 | Group 2 | p-value |
|---|---|---|---|
| Non-neoplastic lesions (n=55) | |||
| Urothelial hyperplasia | 7 | 4 | |
| Brunn nest | 14 | 5 | |
| Cystitis cystica | 5 | 2 | |
| Cystitis glandularis | 9 | 0 | |
| Squamous metaplasia | 8 | 0 | |
| Malakoplakia | 1 | 0 | |
| Sum, No. (%) | 44 (23.9) | 11 (11.7) | <0.001 |
| Cystitis (n=114) | |||
| Acute/chronic cystitis | 21 | 20 | |
| Non-specific cystitis | 22 | 16 | |
| Eosinophilic cystitis | 8 | 5 | |
| Polypoid cystitis | 4 | 3 | |
| Florid proliferative cystitis | 3 | 0 | |
| Follicular cystitis | 3 | 2 | |
| Hemorrhagic cystitis | 1 | 0 | |
| Interstitial cystitis | 1 | 0 | |
| Denuding cystitis | 1 | 0 | |
| Granulomatous cystitis | 0 | 4 | |
| Sum, No. (%) | 64 (34.8) | 50 (53.2) | <0.001 |
| Noninvasive urothelial neoplasms (n=63) | |||
| Urothelial papilloma | 1 | 4 | |
| Urothelial dysplasia | 7 | 3 | |
| Inverted papilloma | 14 | 1 | |
| PUNLMP | 26 | 7 | |
| Sum, No. (%)a) | 22 (12.0) | 8 (8.5) | 0.86 |
| Mesenchymal and other tumors (n=9) | |||
| Myofibroblastic lesion | 3 | 0 | |
| Paraganglioma | 1 | 0 | |
| Smooth muscle tumors | 1 | 0 | |
| Neurofibroma | 1 | 0 | |
| Vascular tumors | 2 | 0 | |
| Lymphoma | 1 | 0 | |
| Sum, No. (%) | 9 (0.5) | 0 | - |
| Others (n=70) | |||
| Sum, No. (%) | 45 (24.5) | 25 (26.6) | - |
| Total sum (n=278)a) | 184 | 94 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download