2. Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021; 19(7):409–424. PMID:
34075212.
3. Wang R, Chen J, Gao K, Wei GW. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021; 113(4):2158–2170. PMID:
34004284.
4. Gupta L, Gasparyan AY, Misra DP, Agarwal V, Zimba O, Yessirkepov M. Information and misinformation on COVID-19: a cross-sectional survey study. J Korean Med Sci. 2020; 35(27):e256. PMID:
32657090.
5. Ahmed S, Gasparyan AY, Zimba O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int. 2021; 41(2):243–256. PMID:
33388969.
6. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020; 94:91–95. PMID:
32173574.
7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020; 323(20):2052–2059. PMID:
32320003.
8. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020; 55(5):2000547. PMID:
32217650.
9. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062. PMID:
32171076.
10. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011; 31(11):1409–1417. PMID:
21800117.
11. AlRyalat SA, Malkawi LW, Momani SM. Comparing bibliometric analysis using PubMed, Scopus, and Web of Science databases. J Vis Exp. 2019; (152):
12. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223):507–513. PMID:
32007143.
13. Gęca T, Wojtowicz K, Guzik P, Góra T. Increased risk of COVID-19 in patients with diabetes mellitus-current challenges in pathophysiology, treatment and prevention. Int J Environ Res Public Health. 2022; 19(11):6555. PMID:
35682137.
14. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018; 34(5):575–584. PMID:
29459239.
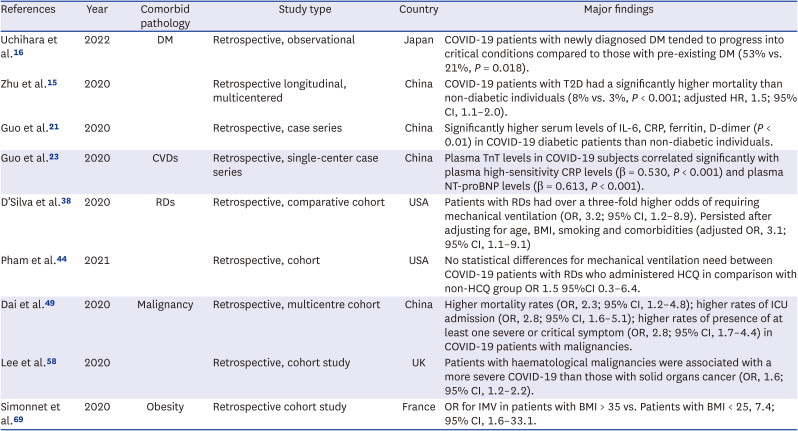
15. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020; 31(6):1068–1077.e3. PMID:
32369736.
16. Uchihara M, Bouchi R, Kodani N, Saito S, Miyazato Y, Umamoto K, et al. Impact of newly diagnosed diabetes on coronavirus disease 2019 severity and hyperglycemia. J Diabetes Investig. 2022; 13(6):1086–1093.
17. Filippatos TD, Liontos A, Papakitsou I, Elisaf MS. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med. 2019; 131(2):82–88. PMID:
30757937.
18. Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020; 8(6):546–550. PMID:
32334646.
19. Assaad M, Hekmat-Joo N, Hosry J, Kassem A, Itani A, Dahabra L, et al. Insulin use in type II diabetic patients: a predictive of mortality in covid-19 infection. Diabetol Metab Syndr. 2022; 14(1):85. PMID:
35725489.
20. Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020; 38(5):781–782. PMID:
32195824.
21. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020; 36(7):e3319. PMID:
32233013.
22. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020; 109(5):531–538. PMID:
32161990.
23. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7):811–818. PMID:
32219356.
24. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(11):1265–1273. PMID:
32730619.
25. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020; 5(7):831–840. PMID:
32219363.
26. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020; 26(7):1017–1032. PMID:
32651579.
27. Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008; 295(6):H2373–H2379. PMID:
18849338.
28. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7):819–824. PMID:
32219357.
29. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020; 5(7):802–810. PMID:
32211816.
30. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020; 142(2):184–186. PMID:
32330083.
31. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020; 116(10):1666–1687. PMID:
32352535.
32. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020; 17(5):259–260. PMID:
32139904.
33. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191:9–14. PMID:
32353746.
34. Ahmed S, Zimba O, Gasparyan AY. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021; 40(7):2611–2619. PMID:
33733315.
35. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020; 220:1–13. PMID:
32299776.
36. Landewé RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020; 79(7):851–858. PMID:
32503854.
37. Cook C, Cox H, Fu X, Zhang Y, Stone JH, Choi HK, et al. Perceived risk and associated shielding behaviors in patients with rheumatoid arthritis during the coronavirus 2019 pandemic. ACR Open Rheumatol. 2021; 3(12):834–841. PMID:
34498436.
38. D’Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020; 79(9):1156–1162. PMID:
32457048.
39. Pablos JL, Galindo M, Carmona L, Lledó A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020; 79(12):1544–1549. PMID:
32796045.
40. Puxeddu I, Ferro F, Bartoloni E, Elefante E, Baldini C, Scirè CA, et al. COVID-19: the new challenge for rheumatologists. One year later. Clin Exp Rheumatol. 2021; 39(1):203–213. PMID:
33555253.
41. Abualfadl E, Ismail F, Shereef RRE, Hassan E, Tharwat S, Mohamed EF, et al. Impact of COVID-19 pandemic on rheumatoid arthritis from a multi-centre patient-reported questionnaire survey: influence of gender, rural-urban gap and north-south gradient. Rheumatol Int. 2021; 41(2):345–353. PMID:
33130920.
42. Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020; 24(8):4539–4547. PMID:
32373993.
43. Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020; 97:396–403. PMID:
32623082.
44. Pham K, Torres H, Satlin MJ, Goyal P, Gulick RM. Failure of chronic hydroxychloroquine in preventing severe complications of COVID-19 in patients with rheumatic diseases. Rheumatol Adv Pract. 2021; 5(1):rkab014. PMID:
33875975.
45. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353(16):1711–1723. PMID:
16236742.
46. Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020; 79(7):859–866. PMID:
32471903.
47. Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020; 39(7):2055–2062. PMID:
32277367.
48. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21(3):335–337. PMID:
32066541.
49. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020; 10(6):783–791. PMID:
32345594.
51. World Health Organization (WHO). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva, Switzerland: World Health Organization;2020.
52. Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020; 26(8):1218–1223. PMID:
32581323.
53. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020; 395(10241):1907–1918. PMID:
32473681.
54. Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020; 21(7):893–903. PMID:
32479790.
55. Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020; 21(7):914–922. PMID:
32539942.
56. Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020; 395(10241):1919–1926. PMID:
32473682.
57. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020; 21(8):1023–1034. PMID:
32702310.
58. Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020; 21(10):1309–1316. PMID:
32853557.
59. Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020; 10(7):935–941. PMID:
32357994.
60. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020; 31(7):894–901. PMID:
32224151.
61. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020; 43(7):1392–1398. PMID:
32409502.
62. Wang J, Xu Y, Zhang X, Wang S, Peng Z, Guo J, et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J Leukoc Biol. 2021; 110(1):9–20. PMID:
33404078.
63. Woo S, Yang H, Kim Y, Lim H, Song HJ, Park KH. Sedentary time and fast-food consumption associated with weight gain during COVID-19 lockdown in children and adolescents with overweight or obesity. J Korean Med Sci. 2022; 37(12):e103. PMID:
35347907.
64. Choi YY, Choi SH, Choi JH, Kim DH, Lee JK, Eun BW, et al. SARS-CoV-2-naïve Korean children and adolescents hospitalized with COVID-19 in 2021. J Korean Med Sci. 2022; 37(42):e303. PMID:
36325607.
65. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006; 13(4):203–210. PMID:
16779465.
66. Dicker D, Bettini S, Farpour-Lambert N, Frühbeck G, Golan R, Goossens G, et al. Obesity and COVID-19: the two sides of the coin. Obes Facts. 2020; 13(4):430–438. PMID:
32659766.
67. Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020; 319(1):E105–E109. PMID:
32459524.
68. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020; 21(11):e13128. PMID:
32845580.
69. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020; 28(7):1195–1199. PMID:
32271993.
70. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020; 71(15):896–897. PMID:
32271368.
71. Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020; 108:154262. PMID:
32422233.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download