This article has been
cited by other articles in ScienceCentral.
Abstract
National cohort data collected during the coronavirus disease 2019 (COVID-19) delta and omicron periods in Korea revealed a lower risk of severe infection in recipients of three doses of the COVID-19 vaccine (adjusted odds ratio [aOR], 0.05–0.08). The risk of death was reduced during the omicron period compared to the delta period (aOR, 0.75; 95% confidence interval, 0.67–0.84).
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Vaccine, Booster
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is characterized by the serial emergence of variants, resulting in continued challenges to public health.
1 The major variants, delta and omicron, have different virulence, transmissibility, and vaccine-evasive properties.
2 Understanding the vaccine effectiveness of severe health outcomes during periods with different dominant SARS-CoV-2 variants is important to guide public health policies. In this study, the risk factors for severe disease and death in patients with coronavirus disease 2019 (COVID-19) in South Korea are investigated to determine the impact of the delta and omicron variants on population health outcomes.
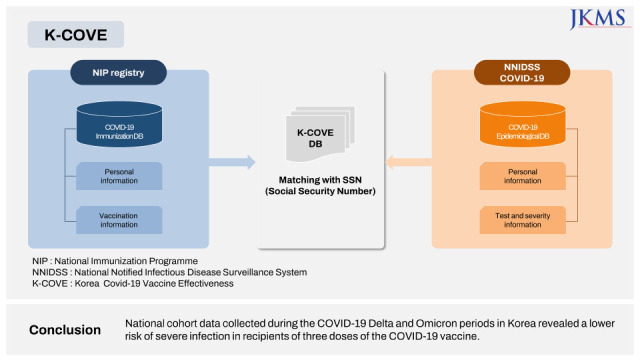
This study is part of the Korea Covid-19 Vaccine Effectiveness (K-COVE) study, which was conducted by the Korea Disease Control and Prevention Agency (KDCA,
Supplementary Fig. 1). National COVID-19 registry data were merged with the vaccine registry data to construct a cohort of patients with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infections reported between July 1, 2021 and January 31, 2022 (the last cases were followed until February 28, 2022), as described in a previous report.
3 The merged database represents the country’s entire population.
Individuals aged < 12 years, foreigners, those immunized with the Ad26.COV2.S vaccine, patients with imported COVID-19, individuals without documented vaccination status, and patients reinfected with COVID-19 were excluded. The monthly percentage of SARS-CoV-2 variants in the patients was determined. For import-related cases, targeted sequencing based on the risk of variant was conducted, whereas for domestic cases, random sampling was conducted to sequence the complete SARS-CoV-2 genome from the available specimens.
4
Severe disease was defined as that in patients who required oxygen supplementation, mechanical ventilation, or intensive care within 28 days of the PCR-confirmed diagnosis. Death in all patients (with and without severe disease) was noted if it occurred within 28 days of the diagnosis based on the follow-up investigations in all reported cases. Unvaccinated individuals were defined as those without COVID-19 immunization history or those who were diagnosed with COVID-19 via PCR < 14 days since the first dose of the vaccine. The vaccine effectiveness was measures against SARS-CoV-2 severe disease or death 14 or more days after the second dose or the third dose.
Patients were stratified based on the interval between the last dose of vaccine and the PCR-confirmed diagnosis of COVID-19 (before 90 days and ≥ 90 days). The type of immunization received was classified as viral vector (ChAdOx1) or mRNA (BNT162b2 and mRNA-1273). A multivariable logistic regression model was constructed with the composite of sex, age (continuous [years] and categorical), area of residence, month of the PCR-confirmed diagnosis, and vaccination status to calculate the adjusted odds ratio (aOR) of severe disease or death.
Anonymized clustered data were shared with the investigators. R software (R Core Team) was used to prepare the data and perform the statistical analyses.
In total, 530,827 patients with PCR-confirmed SARS-CoV-2 infections were included in the analyses (
Supplementary Fig. 2). The delta variant accounted for 59.6–100% of the COVID-19 infections between July and December 2021. In January 2022, 69.4% of the isolates were identified as the omicron variant (
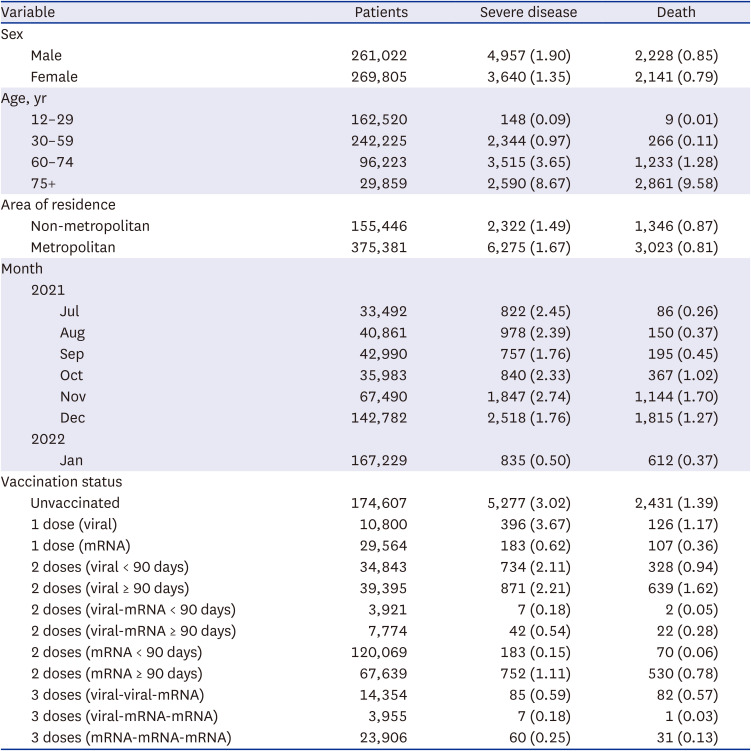
Supplementary Fig. 3). Among unvaccinated patients, 3.02% had severe disease and 1.39% died. Among patients who received three doses of vaccine 0.18–0.59% had severe disease and 0.03–0.57% died (
Table 1).
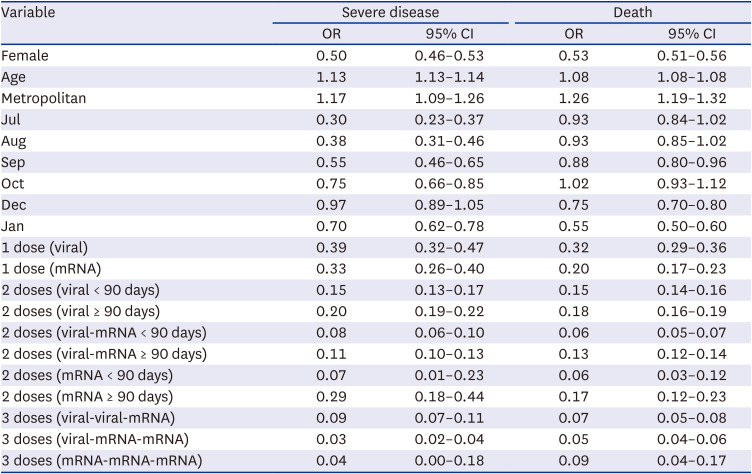
Three doses of mRNA vaccines (aOR, 0.05; 95% confidence interval [CI], 0.04-0.07), two doses of viral vector vaccine followed by an additional dose of mRNA vaccine (aOR, 0.06; 95% CI, 0.05–0.07), and one dose of viral vector vaccine followed by two doses of mRNA vaccines (aOR, 0.08; 95% CI, 0.03–0.15) were associated with lower risks of severe disease (
Fig. 1). The risk of death was lower in January 2022 (aOR, 0.75; 95% CI, 0.67–0.84) than in November 2021. Three doses of mRNA vaccines had the lowest risk of death (aOR, 0.02; 95% CI, 0.01–0.03).
Table 2 shows the multivariable logistic regression model with the composite of sex, age in years, area, month of diagnosis, and vaccination status, to estimate the risk factors for severe disease and death.
The emergence of the omicron variant in January 2022 may have resulted in a lower risk of severe disease and death in patients with COVID-19. Three doses of vaccine significantly protected individuals from critical health outcomes. Previous studies conducted in South Africa,
2 England,
5 and Canada
6 suggested that the clinical severity of the SARS-CoV-2 omicron variant was less than that of the delta variant, which is consistent with the results of the current study. A U.S. study reported a lower risk of severe disease in hospitalized patients with the omicron variant than in those with the delta variant (aOR, 0.61; 95% CI, 0.49–0.77).
7 A Swedish study suggested that patients with the omicron variant had a lower risk of death, though the risk remained high (> 5%) for older people with comorbidities.
8 The results of the current study suggest that the vaccines remain the most important modifiable intervention to protect public health and are consistent with the results of a recent observational cohort study.
9 The findings of the current study highlight the importance of vaccination against COVID-19, especially in people at risk for developing serious illness. The vaccine effectiveness against severe disease decreased as the time from immunization increased. However, any number of vaccine doses and both vaccine types were effective for the protection against death, highlighting the benefits of COVID-19 vaccines.
This study is limited by the retrospective study design. The nature of SARS-CoV-2 outbreaks and public health measures evolved during the study period, especially during January 2022 when the COVID-19 outbreak peaked. The healthcare capacity to care for patients may have differed between July 2021 and January 2022. In addition, severe disease and death data are subject to reporting delays. While a multivariable logistic regression model stratified by vaccinated date, test date, and observation period was used, if the probability to get tested differed between the delta and omicron period, a residual bias may remain. Moreover, other factors, including underlying disease, clinical details, socioeconomic status, and laboratory findings were not included in the analyses, thus posing residual confounding effect to the result. Despite these limitations, this study includes a large population of patients with PCR-confirmed COVID-19 infections in a country that has universal health coverage and relatively equitable access to care. This study includes vaccination health outcome data collected on a national scale, allowing for precise characterization of time periods dominated by specific variants of SARS-CoV-2.
The use of a national cohort allows for a robust analysis to describe the risk factors associated with severe disease and death from COVID-19 in South Korea, and the importance of vaccinations was revealed in this study. The COVID-19 vaccine has been available to children aged 5–11 years in South Korea since March 31, 2022. Based on the disease burden of COVID-19 in this population, the evaluation of the vaccine effectiveness should be expanded to include children in future studies. Understanding the public health benefits of vaccination provides vital information required to emphasize patient awareness among those at risk for severe clinical outcomes.
Ethics statement
This study was conducted as a legally mandated public health investigation under the authority of the Korean Infectious Diseases Control and Prevention Act (No. 12,444 and No. 13,392) and was reviewed by the Korea Disease Control and Prevention Agency Institutional Board Review (IRB No. 2021-12-03-PE-A).
ACKNOWLEDGMENTS
We thank the COVID-19 Vaccination Task Force and Division of National Immunization, Korea Disease Control and Prevention Agency; relevant ministries, including the Ministry of Interior and Safety, Si/Do and Si/Gun/Gu, medical staffs of the health centers, and medical facilities for their efforts in responding to the COVID-19 outbreak. This study was a part of K-COVE (Korea COvid-19 Vaccine Effectiveness) Initiative.
References
1. Lauring AS, Hodcroft EB. Genetic variants of SARS-CoV-2-what do they mean? JAMA. 2021; 325(6):529–531. PMID:
33404586.
2. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022; 399(10323):437–446. PMID:
35065011.
3. Choe YJ, Yi S, Hwang I, Kim J, Park YJ, Cho E, et al. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022; 40(5):691–694. PMID:
35012777.
4. Kim EY, Choe YJ, Park H, Jeong H, Chung JH, Yu J, et al. Community transmission of SARS-CoV-2 Omicron variant, South Korea, 2021. Emerg Infect Dis. 2022; 28(4):898–900. PMID:
35171760.
5. Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022; 399(10332):1303–1312. PMID:
35305296.
6. Ulloa AC, Buchan SA, Daneman N, Brown KA. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. 2022; 327(13):1286–1288. PMID:
35175280.
7. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022; 376:e069761. PMID:
35264324.
8. Kahn F, Bonander C, Moghaddassi M, Rasmussen M, Malmqvist U, Inghammar M, et al. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities - surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill. 2022; 27(9):2200121. PMID:
35241215.
9. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022; 386(19):1804–1816. PMID:
35263534.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
Description of immunization registry linked with epidemiological database to form K-COVE data.K-COVE = Korea Covid-19 Vaccine Effectiveness, COVID-19 = coronavirus disease 2019, NIP = National Immunization Programme, SSN = Social Security Number, NNIDSS = National Notified Infectious Disease Surveillance System.
jkms-38-e87-s001.doc
Supplementary Fig. 3
Percentage of SARS-CoV-2 variants of concerns detected in South Korea, April 2021–January 2022.
jkms-38-e87-s003.doc
Fig. 1
Multivariable logistic regression model with the composite of sex, age categories, area of residence, month of diagnosis, and vaccination status to estimate risk factors for severe disease and death in patients with PCR-confirmed COVID-19 in South Korea, July 2021–January 2022.
PCR = polymerase chain reaction, COVID-19 = coronavirus disease 2019, CI = confidence interval.
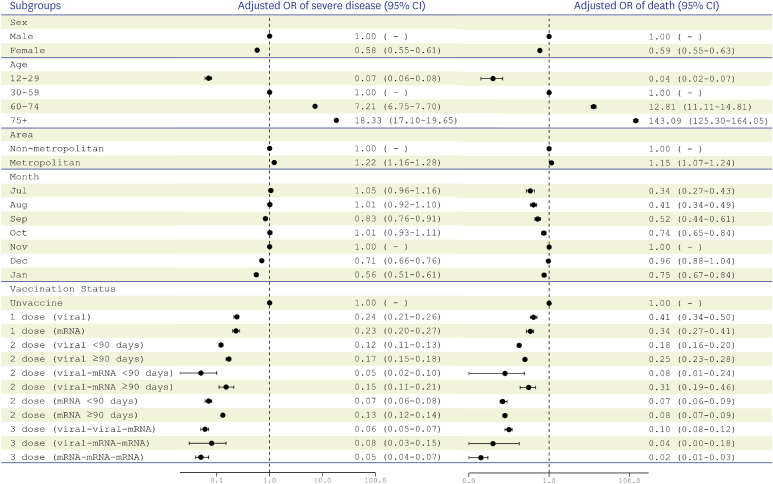
Table 1
Severe disease and death in patients with PCR-confirmed COVID-19
|
Variable |
Patients |
Severe disease |
Death |
|
Sex |
|
|
|
|
Male |
261,022 |
4,957 (1.90) |
2,228 (0.85) |
|
Female |
269,805 |
3,640 (1.35) |
2,141 (0.79) |
|
Age, yr |
|
|
|
|
12–29 |
162,520 |
148 (0.09) |
9 (0.01) |
|
30–59 |
242,225 |
2,344 (0.97) |
266 (0.11) |
|
60–74 |
96,223 |
3,515 (3.65) |
1,233 (1.28) |
|
75+ |
29,859 |
2,590 (8.67) |
2,861 (9.58) |
|
Area of residence |
|
|
|
|
Non-metropolitan |
155,446 |
2,322 (1.49) |
1,346 (0.87) |
|
Metropolitan |
375,381 |
6,275 (1.67) |
3,023 (0.81) |
|
Month |
|
|
|
|
2021 |
|
|
|
|
|
Jul |
33,492 |
822 (2.45) |
86 (0.26) |
|
|
Aug |
40,861 |
978 (2.39) |
150 (0.37) |
|
|
Sep |
42,990 |
757 (1.76) |
195 (0.45) |
|
|
Oct |
35,983 |
840 (2.33) |
367 (1.02) |
|
|
Nov |
67,490 |
1,847 (2.74) |
1,144 (1.70) |
|
|
Dec |
142,782 |
2,518 (1.76) |
1,815 (1.27) |
|
2022 |
|
|
|
|
|
Jan |
167,229 |
835 (0.50) |
612 (0.37) |
|
Vaccination status |
|
|
|
|
Unvaccinated |
174,607 |
5,277 (3.02) |
2,431 (1.39) |
|
1 dose (viral) |
10,800 |
396 (3.67) |
126 (1.17) |
|
1 dose (mRNA) |
29,564 |
183 (0.62) |
107 (0.36) |
|
2 doses (viral < 90 days) |
34,843 |
734 (2.11) |
328 (0.94) |
|
2 doses (viral ≥ 90 days) |
39,395 |
871 (2.21) |
639 (1.62) |
|
2 doses (viral-mRNA < 90 days) |
3,921 |
7 (0.18) |
2 (0.05) |
|
2 doses (viral-mRNA ≥ 90 days) |
7,774 |
42 (0.54) |
22 (0.28) |
|
2 doses (mRNA < 90 days) |
120,069 |
183 (0.15) |
70 (0.06) |
|
2 doses (mRNA ≥ 90 days) |
67,639 |
752 (1.11) |
530 (0.78) |
|
3 doses (viral-viral-mRNA) |
14,354 |
85 (0.59) |
82 (0.57) |
|
3 doses (viral-mRNA-mRNA) |
3,955 |
7 (0.18) |
1 (0.03) |
|
3 doses (mRNA-mRNA-mRNA) |
23,906 |
60 (0.25) |
31 (0.13) |
Table 2
Multivariable logistic regression model with the composite of sex, age in years, area, month of the PCR-confirmation, and vaccination status, to estimate risk factors for severe disease and death in PCR-confirmed COVID-19 case-patients within specified subgroups, South Korea, July 2021–January 2022
|
Variable |
Severe disease |
Death |
|
OR |
95% CI |
OR |
95% CI |
|
Female |
0.50 |
0.46–0.53 |
0.53 |
0.51–0.56 |
|
Age |
1.13 |
1.13–1.14 |
1.08 |
1.08–1.08 |
|
Metropolitan |
1.17 |
1.09–1.26 |
1.26 |
1.19–1.32 |
|
Jul |
0.30 |
0.23–0.37 |
0.93 |
0.84–1.02 |
|
Aug |
0.38 |
0.31–0.46 |
0.93 |
0.85–1.02 |
|
Sep |
0.55 |
0.46–0.65 |
0.88 |
0.80–0.96 |
|
Oct |
0.75 |
0.66–0.85 |
1.02 |
0.93–1.12 |
|
Dec |
0.97 |
0.89–1.05 |
0.75 |
0.70–0.80 |
|
Jan |
0.70 |
0.62–0.78 |
0.55 |
0.50–0.60 |
|
1 dose (viral) |
0.39 |
0.32–0.47 |
0.32 |
0.29–0.36 |
|
1 dose (mRNA) |
0.33 |
0.26–0.40 |
0.20 |
0.17–0.23 |
|
2 doses (viral < 90 days) |
0.15 |
0.13–0.17 |
0.15 |
0.14–0.16 |
|
2 doses (viral ≥ 90 days) |
0.20 |
0.19–0.22 |
0.18 |
0.16–0.19 |
|
2 doses (viral-mRNA < 90 days) |
0.08 |
0.06–0.10 |
0.06 |
0.05–0.07 |
|
2 doses (viral-mRNA ≥ 90 days) |
0.11 |
0.10–0.13 |
0.13 |
0.12–0.14 |
|
2 doses (mRNA < 90 days) |
0.07 |
0.01–0.23 |
0.06 |
0.03–0.12 |
|
2 doses (mRNA ≥ 90 days) |
0.29 |
0.18–0.44 |
0.17 |
0.12–0.23 |
|
3 doses (viral-viral-mRNA) |
0.09 |
0.07–0.11 |
0.07 |
0.05–0.08 |
|
3 doses (viral-mRNA-mRNA) |
0.03 |
0.02–0.04 |
0.05 |
0.04–0.06 |
|
3 doses (mRNA-mRNA-mRNA) |
0.04 |
0.00–0.18 |
0.09 |
0.04–0.17 |







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download