Patient characteristics
The clinical features of 45,860 patients (4,223 patients with NENs and 41,637 patients with non-NECs), including the age at diagnosis, sex, and metastatic rates, are summarized in
Table 1,
Supplementary Tables 1,
2,
3,
4,
5,
6,
7,
8, and
Fig. 1. Colorectal NET (n = 623) was the most common NET in this study, followed by gastric NET (n = 134), pulmonary NET (n = 114), small intestinal NET (n = 99), and pancreatic NET (n = 50). Among 623 colorectal NET cases, 589 cases (94.5%) were rectal NET.
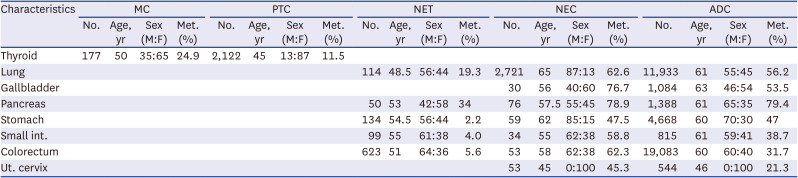
Table 1
Summary of patients’ clinical characteristics, including the median age at diagnosis, sex ratio and metastasis rate
|
Characteristics |
MC |
PTC |
NET |
NEC |
ADC |
|
No. |
Age, yr |
Sex (M:F) |
Met. (%) |
No. |
Age, yr |
Sex (M:F) |
Met. (%) |
No. |
Age, yr |
Sex (M:F) |
Met. (%) |
No. |
Age, yr |
Sex (M:F) |
Met. (%) |
No. |
Age, yr |
Sex (M:F) |
Met. (%) |
|
Thyroid |
177 |
50 |
35:65 |
24.9 |
2,122 |
45 |
13:87 |
11.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Lung |
|
|
|
|
|
|
|
|
114 |
48.5 |
56:44 |
19.3 |
2,721 |
65 |
87:13 |
62.6 |
11,933 |
61 |
55:45 |
56.2 |
|
Gallbladder |
|
|
|
|
|
|
|
|
|
|
|
|
30 |
56 |
40:60 |
76.7 |
1,084 |
63 |
46:54 |
53.5 |
|
Pancreas |
|
|
|
|
|
|
|
|
50 |
53 |
42:58 |
34 |
76 |
57.5 |
55:45 |
78.9 |
1,388 |
61 |
65:35 |
79.4 |
|
Stomach |
|
|
|
|
|
|
|
|
134 |
54.5 |
56:44 |
2.2 |
59 |
62 |
85:15 |
47.5 |
4,668 |
60 |
70:30 |
47 |
|
Small int. |
|
|
|
|
|
|
|
|
99 |
55 |
61:38 |
4.0 |
34 |
55 |
62:38 |
58.8 |
815 |
61 |
59:41 |
38.7 |
|
Colorectum |
|
|
|
|
|
|
|
|
623 |
51 |
64:36 |
5.6 |
53 |
58 |
62:38 |
62.3 |
19,083 |
60 |
60:40 |
31.7 |
|
Ut. cervix |
|
|
|
|
|
|
|
|
|
|
|
|
53 |
45 |
0:100 |
45.3 |
544 |
46 |
0:100 |
21.3 |
Fig. 1
Comparison of the patients’ median age at diagnosis (years), proportion of male patients (%) and metastasis rate (%).
MTC = medullary thyroid carcinoma, PTC = papillary thyroid carcinoma, NET = neuroendocrine tumor, NEC = neuroendocrine carcinoma, ADC = adenocarcinoma, GB = gallbladder, Ut. cervix = uterine cervix.
Only statistically significant comparisons are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
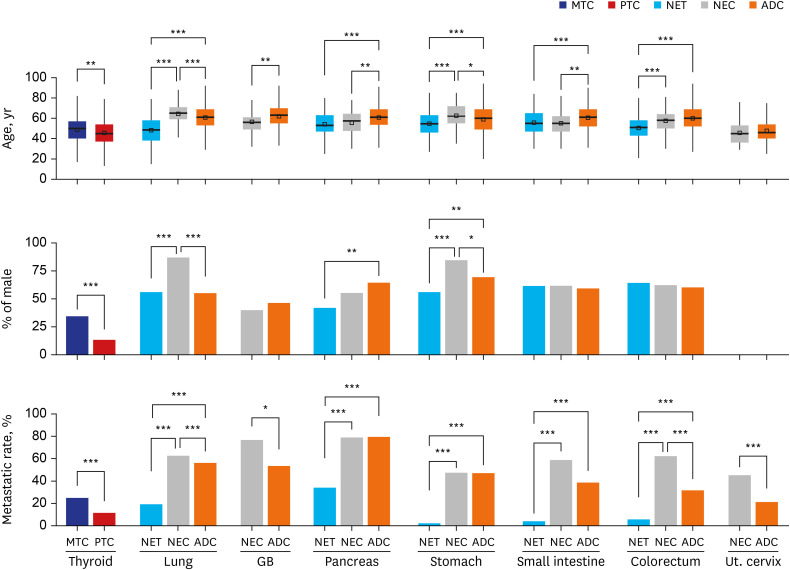
The median ages of patients with NETs were significantly (all P < 0.001) younger than those of patients with ADCs in all primary sites. In contrast, the median ages of patients with NECs showed a variable relationship with the median ages of patients with ADCs, as patients with gallbladder, pancreas, and small intestine involvement were significantly younger, similar ages were observed for patients with colorectum and uterine cervix involvement, and patients with lung and stomach involvement were significantly older. The median age of patients with MTC was also significantly (P = 0.001) older than the median age of patients with PTC.
Compared to median age, differences in sex distributions were not significant. Patients with NETs and NECs generally showed a male predominance, except for those with pancreatic NET and gallbladder NEC. Significantly more females (P = 0.001) were diagnosed with pancreatic NETs than with pancreatic ADCs. Gallbladder NEC and gallbladder ADC both showed a slight female predominance. Additionally, patients with pulmonary NEC and gastric NEC were significantly more likely to be male than patients with NETs and ADCs of each primary organ.
Overall, the metastasis rates of NETs were significantly lower than those of NECs or ADCs in all primary organs. Pancreatic NETs showed the highest metastasis rate (34.0%), followed by pulmonary NETs (19.3%), colorectal NETs (5.6%), small intestinal NETs (4.0%), and gastric NETs (2.2%). In contrast, the metastasis rates of NECs were similar to or significantly higher than those of ADCs. Among NECs, pancreatic NECs showed the highest metastasis rate (78.9%), followed by gallbladder NECs (76.7%), pulmonary SCLCs (63.3%), colorectal NECs (62.3%), small intestinal NECs (58.8%), pulmonary LCNECs (57.7%), gastric NECs (47.5%), and uterine cervical NECs (45.3%). The metastasis rate of MTC (24.9%) was also significantly (P < 0.001) higher than that of PTC (11.5%).
Epidemiology of metastasis
A total of 39,609 records of metastases were identified for the 45,860 patients included in this study. The most common site of metastasis was the liver (7,783/39,609, 19.7%), followed by distant LNs (6,680/39,609, 16.9%), lung (6,230/39,609, 15.7%), bone (4,984/39,609, 12.6%), central nervous system (CNS) (3,416/39,609, 8.6%), peritoneum (3,229/39,609, 8.2%), and pleura (2,793/39,609, 7.0%). Metastasis to these organs accounted for 88.7% (35,115/39,609) of all metastases. Among the remaining organs, a few significant differences in organotrophic metastasis rates were also observed among NETs, NECs, and non-NENs. However, metastasis rates were too low to have clinical significance, despite their statistical significance. For example, splenic organotrophic metastasis was significantly different (
P < 0.001) between pulmonary SCLC (5/1,502, 0.3%) and LCNEC (7/202, 3.5%), although these rates were too low to have clinical significance. We describe the details of metastases, including metastases to the remaining organs, in the
Supplementary Tables 1,
2,
3,
4,
5,
6,
7,
8. We subsequently focused on comparing the patterns of organotrophic metastasis to the liver, distant LNs, lung, bone, CNS, peritoneum, and pleura (
Fig. 2).
Fig. 2
Comparison of metastatic patterns between patients with NENs and patients with non-NENs in various primary sites. Each percentage indicates the percentage of patients with metastasis to the indicated organ divided by the total number of patients with metastasis. For thyroid cancer, patients with LN metastasis alone were excluded from the total number of patients with metastasis.
NEN = neuroendocrine neoplasm, MTC = medullary thyroid carcinoma, PTC = papillary thyroid carcinoma, NET = neuroendocrine tumor, NEC = neuroendocrine carcinoma, ADC = adenocarcinoma, GB = gallbladder, Ut. cervix = uterine cervix, LN = lymph node, CNS = central nervous system.
Only statistically significant comparisons are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
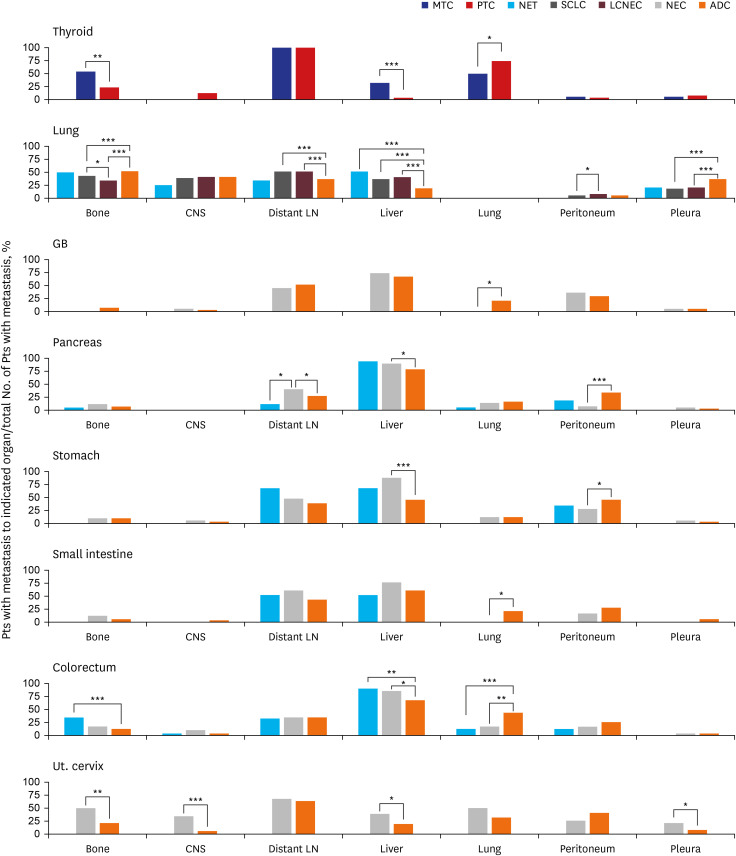
Comparison of metastatic patterns
NETs and NECs from the same primary sites showed no significant differences in metastatic patterns, with three exceptions. The bone organotrophic metastasis rate of pulmonary SCLC (589/1,502, 39.2%) was significantly higher (P = 0.028) than that of pulmonary LCNEC (63/202, 31.2%). The peritoneal organotrophic metastasis rate of pulmonary LCNEC (9/202, 4.5%) was significantly higher (P = 0.037) than that of pulmonary SCLC (29/1,502, 1.9%). The distant LN organotrophic metastasis rate of pancreatic NEC (24/60, 40.0%) was significantly higher (P = 0.030) than that of pancreatic NET (2/17, 11.8%).
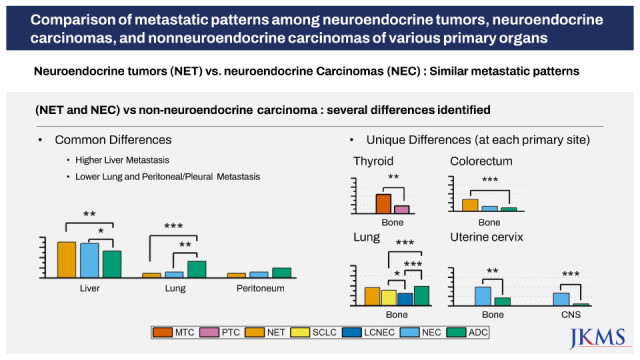
When we compared the metastatic patterns of NENs and non-NENs in various primary sites, we observed two types of differences. One type entailed differences shared by NENs from most primary sites; the other was differences specific to each organ.
NENs showed higher liver organotrophic metastasis rates, lower pleural/peritoneal organotrophic metastasis rates and lower lung organotrophic metastasis rates than matched non-NENs in most primary sites.
Higher liver organotrophic metastasis rates than those of matched non-NENs were identified for MTC (7/22, 31.8%, P < 0.001), pulmonary NET (11/22, 50%, P < 0.001), pulmonary SCLC (508/1,502, 33.8%, P < 0.001), pulmonary LCNEC (78/202, 38.6%, P < 0.001), pancreatic NEC (54/60, 90%, P = 0.022), gastric NEC (24/28, 85.7%, P < 0.001), colorectal NET (31/35, 88.6%, P = 0.005), colorectal NEC (28/33, 84.8%, P = 0.026), and uterine cervical NEC (22/116, 19%, P = 0.047). However, the liver organotrophic metastasis rates of gallbladder NEC (17/23, 73.9%), pancreatic NET (16/17, 94.1%), gastric NET (2/3, 66.7%), small intestinal NET (2/4, 50%) and small intestinal NEC (15/20, 75%) were not significantly different from those of matched non-NENs of the same primary tissue (P = 0.462, P = 0.141, P = 0.581, P = 0.652 and P = 0.240, respectively), probably due to the small numbers of patients included in this study. Interestingly, the liver organotrophic metastasis rate of NENs increased in proportion to the increase in the liver metastasis rate of non-NENs from the same primary site.
Lower peritoneal organotrophic metastasis rates than those of matched non-NENs were identified for pancreatic NEC (5/60, 8.3%, P < 0.001) and gastric NEC (7/28, 25.0%, P = 0.041). The remaining intra-abdominal NENs did not show statistically significant differences in peritoneal organotrophic metastasis rates. However, colorectal NEN (sum of NET and NEC) (9/68, 13.2%) showed significantly lower peritoneal organotrophic metastasis rates (P = 0.028) than colorectal ADC (1,496/6,040, 24.8%), although neither colorectal NET (4/35, 11.4%) nor colorectal NEC (5/33, 15.2%) showed statistically significant differences (P = 0.068 and P = 0.202) compared to colorectal ADC. Therefore, we suspect that the small number of cases might explain the lack of significant differences. Additionally, pulmonary SCLC (250/1,502, 16.6%) and LCNEC (38/202, 18.8%) showed lower pleural organotrophic metastasis rates (both P < 0.001) than pulmonary ADC (2,234/6,701, 33.3%). None of the pulmonary NETs (0/22, 0%) exhibited pleural metastasis.
Lower lung organotrophic metastasis rates than those of matched non-NENs were identified for MTC (11/22, 50.0%, P < 0.038), colorectal NET (4/35, 11.4%, P < 0.001), and colorectal NEC (5/33, 15.2%, P = 0.002). No gallbladder NEC (0/23), gastric NET (0/3), small intestinal NET (0/4), or small intestinal NEC (0/20) underwent lung metastasis. However, the lung organotrophic metastasis rates of pancreatic NET (1/17, 5.9%), pancreatic NEC (8/60, 13.3%), gastric NEC (3/28, 10.7%), and uterine cervical NEC (12/24, 50.0%) were not significantly different from those of matched non-NENs from the same primary tissue (P = 0.494, P = 0.701, P = 1.000, and P = 0.091, respectively).
In addition to previously described differences shared by various primary sites, we observed several significant differences in metastatic patterns between NENs and non-NENs specific to each organ. In the thyroid, MTC (12/22, 54.5%) exhibited a significantly higher bone organotrophic metastasis rate (P < 0.001) than PTC (16/72, 22.2%). In the lung, SCLC (589/1,502, 39.2%) and LCNEC (63/202, 31.2%) showed significantly lower bone organotrophic metastasis rates (both P < 0.001) than ADC (3,248/6,701, 48.5%). Significantly higher distant LN organotrophic metastasis rates were also observed for SCLC (729/1,502, 48.5%) and LCNEC (99/202, 49.0%) than ADC (2297/6701, 37.1%). In the pancreas, NEC (24/60, 40.0%) exhibited a significantly higher distant LN organotrophic metastasis rate (P = 0.037) than ADC (304/1,102, 27.6%). In the colorectum, NET (12/35, 34.3%) showed a significantly higher bone organotrophic metastasis rate (P < 0.001) than ADC (641/6,040, 10.6%). In the uterine cervix, NEC (12/24, 50.0% and 8/24, 33.3%, respectively) had significantly higher bone and CNS organotrophic metastasis rates (P = 0.003 and P < 0.001, respectively) than ADC (24/116, 20.7% and 6/116, 5.2%, respectively). Uterine cervical NEC (5/24, 20.8%) also had a significantly higher pleural organotrophic metastasis rate (P = 0.048) than ADC (8/116, 6.9%).







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download