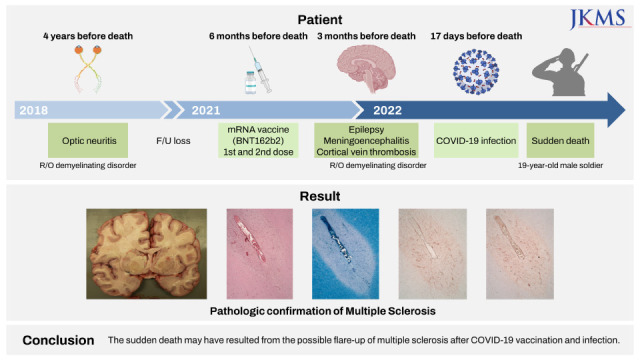
INTRODUCTION
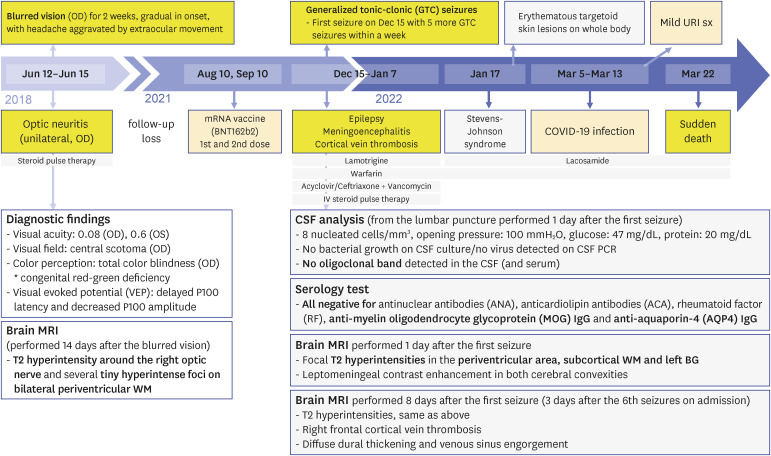
CASE DESCRIPTION
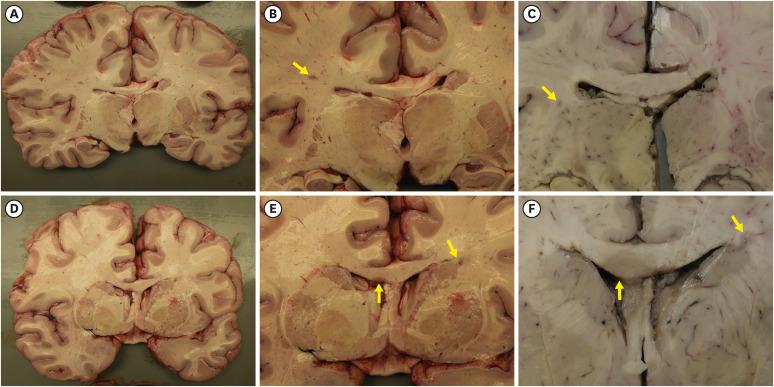 | Fig. 2Gross findings of the brain.(A-F) Coronal sections of the brain revealed multifocal, well-demarcated, gray-tan, demyelinated plaques (indicated by yellow arrows) in the deep white matter (B, C), corpus callosum and periventricular area (E, F). (B, E) are close-up photographs of sections in (A, D) respectively. The demyelinated plaques are different in their size and shape (B, C, E, and F). Sections in (C, F) are formalin-fixed. The venocentric distribution of the demyelinated plaques is grossly identified.
|
 | Fig. 3Gross-microscopic correlation of the multifocal lesions of the brain.The demyelinated plaque shown in (A-D) located at periventricular area and abuts basal ganglia. Local infiltrates of macrophages and lymphocytes are present in the plaque (B). LFB stain revealed myelin loss in the plaque, and a finger-like appearance of perivascular demyelination (C). Gliotic fibers are densely condensed within the plaques (D). The demyelinated plaque shown in (E-H) is hypercellular (F), sharply demarcated (G) and contains abundant macrophages and CD45-positive perivascular lymphocytic infiltrations in the plaque (H). The demyelinated plaque shown in (I-L) is a chronic active plaque and consists of central hypocellular inactive center and peripheral rim of macrophage. The macrophage infiltration as shown by CD68 immunostain is not evenly distributed but located preferentially in the peripheral edge of the plaque forming a “rim” (L).
Types of staining: (B, F, and J) H&E; (C, G and K) LFB; (D, H, and L) IHC staining for GFAP, CD45 and CD68. Original magnification: (B-D) ×25; (F-H) ×25; (J-L) ×25.
H&E = hematoxylin and eosin, LFB = Luxol fast blue, IHC = immunohistochemical, GFAP = Glial fibrillary acidic protein.
|
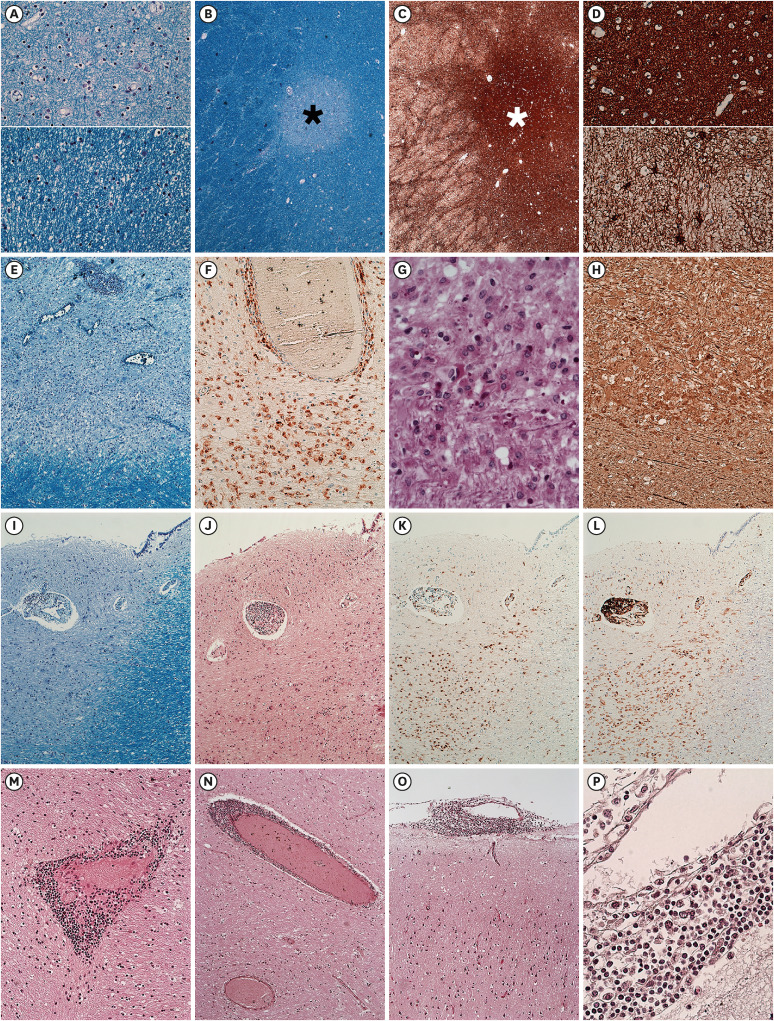 | Fig. 4Demyelinating plaque of different stages.The upper (A) and upper (D) correspond to the center of the plaques, (black asterisk of B and white asterisk of C) while the lower (A) and lower (D) correspond to the peri-plaque area. The center of the plaque shows diminished but not absent myelin fiber (A) and dense gliotic fiber (D). The lesion shown in (E-H) has abundant and evenly distributed macrophages in the plaque. The myelin loss of the plaque is identified (E). CD68-positive macrophages are abundant in the plaque (F). PAS-positive debris in the macrophage are identified (G). Immunohistochemical stain for phosphorylated NF revealed the relative axonal preservation in the plaque (H). The lesion shown in (I-L) abuts the lateral ventricle. Relatively well-defined demyelination is observed and highlighted on LFB (I-J). A peripheral rim of CD68-positive macrophage is observed (K). CD45-positive lymphocytes are located in the edge of the plaque (L). Perivascular lymphocytic cuffing in the parenchyma was frequently observed (M, N) along with lymphocytic infiltration in the leptomeninges (O, P).
Types of staining: (A, B, E, and I) LFB; (C, D) GFAP; (F, K) CD68; (G) PAS; (H) phosphorylated NF; (J, M, N, O, and P) H&E; (L) CD45. Original magnification: (A) ×50; (B, C) ×25; (D) ×50; (E) ×50; (F) ×200; (G) ×400; (H) ×200; (I-L) ×25; (M) ×200; (N, O) ×100; (P) ×400.
LFB = Luxol fast blue, GFAP = Glial fibrillary acidic protein, PAS = Periodic acid Schiff, NF = neurofilament, H&E = hematoxylin and eosin.
|
Ethics statement
DISCUSSION
Table 1
Demographic and clinical features of previously reported demyelinating disease relapse cases following COVID-19 vaccination
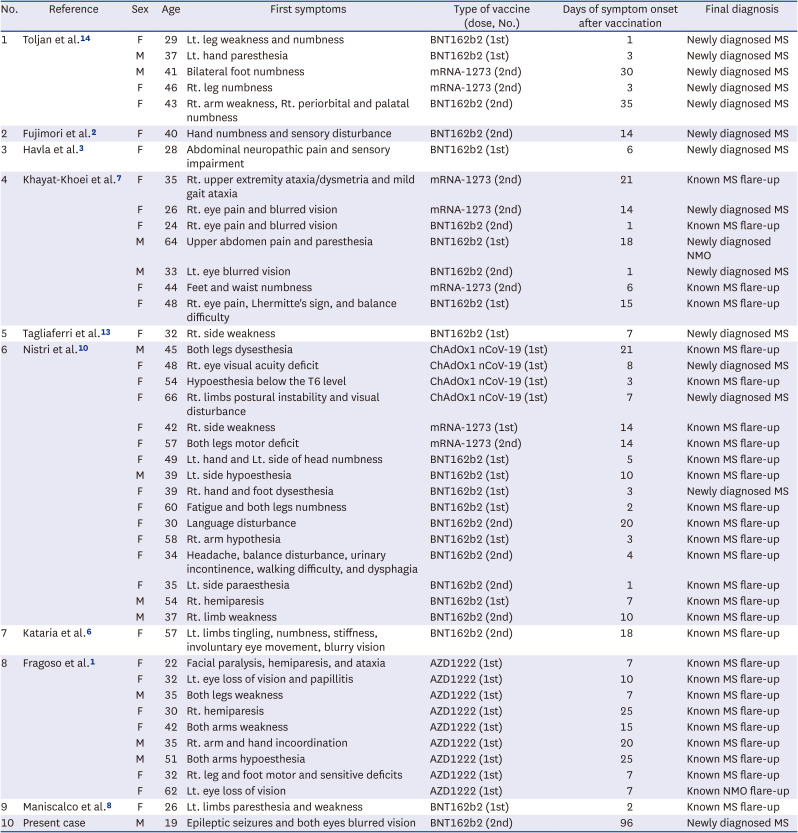
| No. | Reference | Sex | Age | First symptoms | Type of vaccine (dose, No.) | Days of symptom onset after vaccination | Final diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | Toljan et al.14 | F | 29 | Lt. leg weakness and numbness | BNT162b2 (1st) | 1 | Newly diagnosed MS |
| M | 37 | Lt. hand paresthesia | BNT162b2 (1st) | 3 | Newly diagnosed MS | ||
| M | 41 | Bilateral foot numbness | mRNA-1273 (2nd) | 30 | Newly diagnosed MS | ||
| F | 46 | Rt. leg numbness | mRNA-1273 (2nd) | 3 | Newly diagnosed MS | ||
| F | 43 | Rt. arm weakness, Rt. periorbital and palatal numbness | BNT162b2 (2nd) | 35 | Newly diagnosed MS | ||
| 2 | Fujimori et al.2 | F | 40 | Hand numbness and sensory disturbance | BNT162b2 (2nd) | 14 | Newly diagnosed MS |
| 3 | Havla et al.3 | F | 28 | Abdominal neuropathic pain and sensory impairment | BNT162b2 (1st) | 6 | Newly diagnosed MS |
| 4 | Khayat-Khoei et al.7 | F | 35 | Rt. upper extremity ataxia/dysmetria and mild gait ataxia | mRNA-1273 (2nd) | 21 | Known MS flare-up |
| F | 26 | Rt. eye pain and blurred vision | mRNA-1273 (2nd) | 14 | Newly diagnosed MS | ||
| F | 24 | Rt. eye pain and blurred vision | BNT162b2 (2nd) | 1 | Known MS flare-up | ||
| M | 64 | Upper abdomen pain and paresthesia | BNT162b2 (1st) | 18 | Newly diagnosed NMO | ||
| M | 33 | Lt. eye blurred vision | BNT162b2 (2nd) | 1 | Newly diagnosed MS | ||
| F | 44 | Feet and waist numbness | mRNA-1273 (2nd) | 6 | Known MS flare-up | ||
| F | 48 | Rt. eye pain, Lhermitte's sign, and balance difficulty | BNT162b2 (1st) | 15 | Known MS flare-up | ||
| 5 | Tagliaferri et al.13 | F | 32 | Rt. side weakness | BNT162b2 (1st) | 7 | Newly diagnosed MS |
| 6 | Nistri et al.10 | M | 45 | Both legs dysesthesia | ChAdOx1 nCoV-19 (1st) | 21 | Known MS flare-up |
| F | 48 | Rt. eye visual acuity deficit | ChAdOx1 nCoV-19 (1st) | 8 | Newly diagnosed MS | ||
| F | 54 | Hypoesthesia below the T6 level | ChAdOx1 nCoV-19 (1st) | 3 | Known MS flare-up | ||
| F | 66 | Rt. limbs postural instability and visual disturbance | ChAdOx1 nCoV-19 (1st) | 7 | Newly diagnosed MS | ||
| F | 42 | Rt. side weakness | mRNA-1273 (1st) | 14 | Known MS flare-up | ||
| F | 57 | Both legs motor deficit | mRNA-1273 (2nd) | 14 | Known MS flare-up | ||
| F | 49 | Lt. hand and Lt. side of head numbness | BNT162b2 (1st) | 5 | Known MS flare-up | ||
| M | 39 | Lt. side hypoesthesia | BNT162b2 (1st) | 10 | Known MS flare-up | ||
| F | 39 | Rt. hand and foot dysesthesia | BNT162b2 (1st) | 3 | Newly diagnosed MS | ||
| F | 60 | Fatigue and both legs numbness | BNT162b2 (1st) | 2 | Known MS flare-up | ||
| F | 30 | Language disturbance | BNT162b2 (2nd) | 20 | Known MS flare-up | ||
| F | 58 | Rt. arm hypothesia | BNT162b2 (1st) | 3 | Known MS flare-up | ||
| F | 34 | Headache, balance disturbance, urinary incontinence, walking difficulty, and dysphagia | BNT162b2 (2nd) | 4 | Known MS flare-up | ||
| F | 35 | Lt. side paraesthesia | BNT162b2 (2nd) | 1 | Known MS flare-up | ||
| M | 54 | Rt. hemiparesis | BNT162b2 (1st) | 7 | Known MS flare-up | ||
| M | 37 | Rt. limb weakness | BNT162b2 (2nd) | 10 | Known MS flare-up | ||
| 7 | Kataria et al.6 | F | 57 | Lt. limbs tingling, numbness, stiffness, involuntary eye movement, blurry vision | BNT162b2 (2nd) | 18 | Known MS flare-up |
| 8 | Fragoso et al.1 | F | 22 | Facial paralysis, hemiparesis, and ataxia | AZD1222 (1st) | 7 | Known MS flare-up |
| F | 32 | Lt. eye loss of vision and papillitis | AZD1222 (1st) | 10 | Known MS flare-up | ||
| M | 35 | Both legs weakness | AZD1222 (1st) | 7 | Known MS flare-up | ||
| F | 30 | Rt. hemiparesis | AZD1222 (1st) | 25 | Known MS flare-up | ||
| F | 42 | Both arms weakness | AZD1222 (1st) | 15 | Known MS flare-up | ||
| M | 35 | Rt. arm and hand incoordination | AZD1222 (1st) | 20 | Known MS flare-up | ||
| M | 51 | Both arms hypoesthesia | AZD1222 (1st) | 25 | Known MS flare-up | ||
| F | 32 | Rt. leg and foot motor and sensitive deficits | AZD1222 (1st) | 7 | Known MS flare-up | ||
| F | 62 | Lt. eye loss of vision | AZD1222 (1st) | 7 | Known NMO flare-up | ||
| 9 | Maniscalco et al.8 | F | 26 | Lt. limbs paresthesia and weakness | BNT162b2 (1st) | 2 | Known MS flare-up |
| 10 | Present case | M | 19 | Epileptic seizures and both eyes blurred vision | BNT162b2 (2nd) | 96 | Newly diagnosed MS |
Table 2
Demographic and clinical features of previously reported demyelinating disease relapse cases following COVID-19 infection
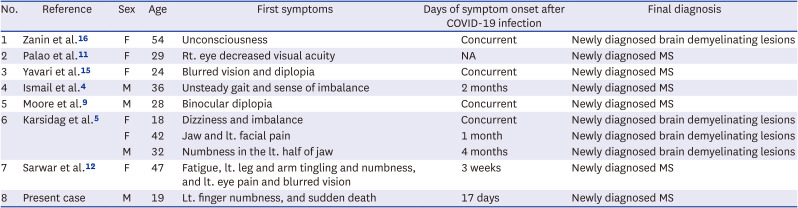
| No. | Reference | Sex | Age | First symptoms | Days of symptom onset after COVID-19 infection | Final diagnosis |
|---|---|---|---|---|---|---|
| 1 | Zanin et al.16 | F | 54 | Unconsciousness | Concurrent | Newly diagnosed brain demyelinating lesions |
| 2 | Palao et al.11 | F | 29 | Rt. eye decreased visual acuity | NA | Newly diagnosed MS |
| 3 | Yavari et al.15 | F | 24 | Blurred vision and diplopia | Concurrent | Newly diagnosed MS |
| 4 | Ismail et al.4 | M | 36 | Unsteady gait and sense of imbalance | 2 months | Newly diagnosed MS |
| 5 | Moore et al.9 | M | 28 | Binocular diplopia | Concurrent | Newly diagnosed MS |
| 6 | Karsidag et al.5 | F | 18 | Dizziness and imbalance | Concurrent | Newly diagnosed brain demyelinating lesions |
| F | 42 | Jaw and lt. facial pain | 1 month | Newly diagnosed brain demyelinating lesions | ||
| M | 32 | Numbness in the lt. half of jaw | 4 months | Newly diagnosed brain demyelinating lesions | ||
| 7 | Sarwar et al.12 | F | 47 | Fatigue, lt. leg and arm tingling and numbness, and lt. eye pain and blurred vision | 3 weeks | Newly diagnosed MS |
| 8 | Present case | M | 19 | Lt. finger numbness, and sudden death | 17 days | Newly diagnosed MS |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download