1. Carpenter MB, Sutin J. Human Neuroanatomy. 8th ed. Baltimore, MD, USA: Williams & Wilkins;1983.
2. Noback CR, Strominger NL, Demarest RJ, Ruggiero DA. Brainstem: medulla, pons, and midbrain. Noback CR, Strominger NL, Demarest RJ, Ruggiero DA, editors. The Human Nervous System: Structure and Function. Totowa, NJ, USA: Humana Press;2005. p. 219–242.
3. Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006; 24(8):1266–1272. PMID:
16525181.
4. Adil SM, Calabrese E, Charalambous LT, Cook JJ, Rahimpour S, Atik AF, et al. A high-resolution interactive atlas of the human brainstem using magnetic resonance imaging. Neuroimage. 2021; 237:118135. PMID:
33951517.
5. Bianciardi M, Toschi N, Eichner C, Polimeni JR, Setsompop K, Brown EN, et al. In vivo functional connectome of human brainstem nuclei of the ascending arousal, autonomic, and motor systems by high spatial resolution 7-Tesla fMRI. Magn Reson Mater Biol Phys Med. 2016; 29(3):451–462.
6. Nguyen TH, Vaussy A, Le Gaudu V, Aboab J, Espinoza S, Curajos I, et al. The brainstem in multiple sclerosis: MR identification of tracts and nuclei damage. Insights Imaging. 2021; 12(1):151. PMID:
34674050.
7. Rushmore RJ, Wilson-Braun P, Papadimitriou G, Ng I, Rathi Y, Zhang F, et al. 3D exploration of the brainstem in 50-micron resolution MRI. Front Neuroanat. 2020; 14:40. PMID:
33071761.
8. Singh K, Cauzzo S, García-Gomar MG, Stauder M, Vanello N, Passino C, et al. Functional connectome of arousal and motor brainstem nuclei in living humans by 7 Tesla resting-state fMRI. Neuroimage. 2022; 249:118865. PMID:
35031472.
9. Coulombe V, Saikali S, Goetz L, Takech MA, Philippe É, Parent A, et al. A topographic atlas of the human brainstem in the ponto-mesencephalic junction plane. Front Neuroanat. 2021; 15:627656. PMID:
34483849.
10. DeArmond SJ, Fusco MM, Dewey MM. Structure of the Human Brain: A Photographic Atlas. 3rd ed. New York, NY, USA: Oxford University Press, Inc.;1989.
11. Leigh RJ. From Nissl stains to modern concepts of brainstem function. Brain. 2014; 138(2):501–503.
12. Sclocco R, Beissner F, Bianciardi M, Polimeni JR, Napadow V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage. 2018; 168:412–426. PMID:
28232189.
13. Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM. Duvernoy's Atlas of the Human Brain Stem and Cerebellum: High-Field MRI, Surface Anatomy, Internal Structure, Vascularization and 3 D Sectional Anatomy. Berlin, Germany: Springer Verlag;2009.
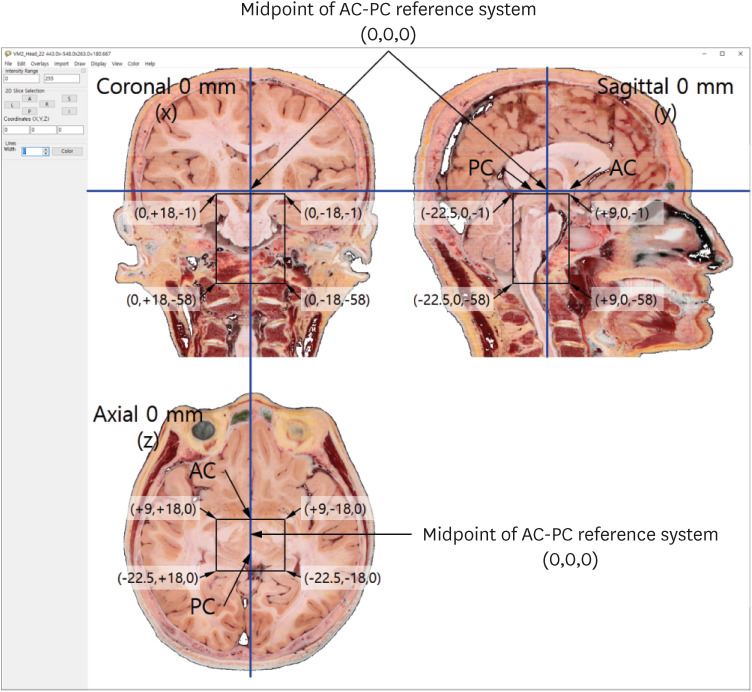
14. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. 1st ed. Stuttgart, Germany: Thieme Stuttgart;1988.
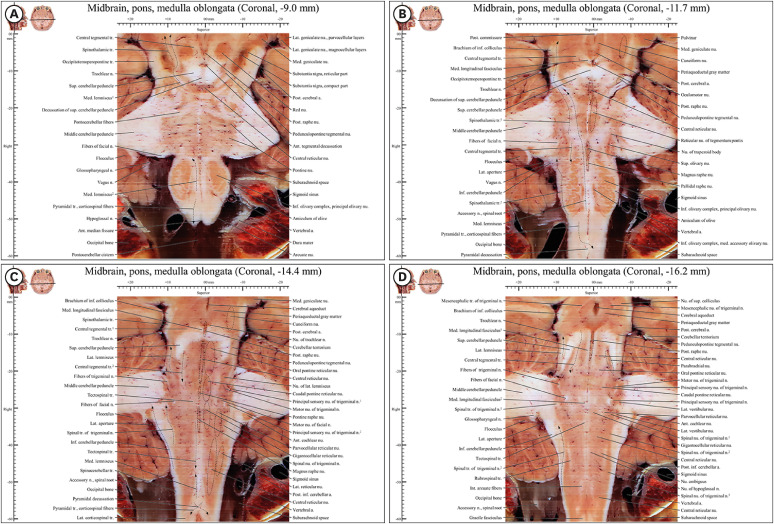
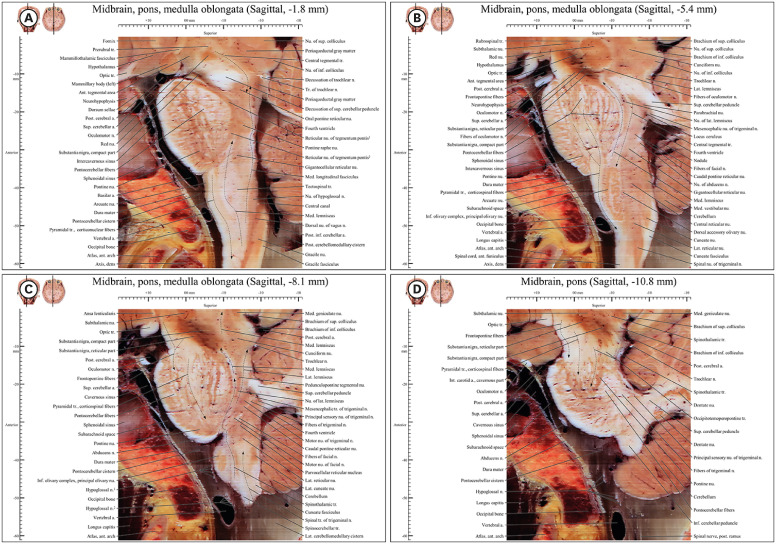
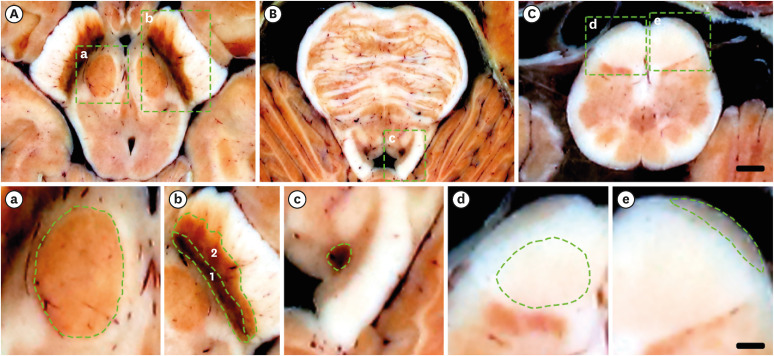
15. Park JS, Chung MS, Park HS, Shin DS, Har DH, Cho ZH, et al. A proposal of new reference system for the standard axial, sagittal, coronal planes of brain based on the serially-sectioned images. J Korean Med Sci. 2010; 25(1):135–141. PMID:
20052359.
16. You Y, Kim CY, Kim SK, Chung BS, Har D, Choi J, et al. Advanced-sectioned images obtained by microsectioning of the entire male body. Clin Anat. 2022; 35(1):79–86. PMID:
34591338.
17. Park JS, Chung MS, Hwang SB, Lee YS, Har DH, Park HS. Visible Korean human: improved serially sectioned images of the entire body. IEEE Trans Med Imaging. 2005; 24(3):352–360. PMID:
15754985.
18. Chung BS, Park JS. Real-color volume models made from real-color sectioned images of Visible Korean. J Korean Med Sci. 2019; 34(10):e86. PMID:
30886552.
19. Paxinos G, Huang XF. Atlas of the Human Brainstem. San Diego, CA, USA: Elsevier Inc.;1995.
20. Federative Committee on Anatomical Terminology. Terminologia Anatomica: International Anatomical Terminology. 1st ed. New York, NY, USA: Thieme;1998.
21. Kiernan JA, Rajakumar R. Barr’s the Human Nervous System: An Anatomical Viewpoint. 10th ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins;2004.
22. Park HS, Chung MS, Shin DS, Jung YW, Park JS. Accessible and informative sectioned images, color-coded images, and surface models of the ear. Anat Rec (Hoboken). 2013; 296(8):1180–1186. PMID:
23713007.
23. Park HS, Chung MS, Shin DS, Jung YW, Park JS. Whole courses of the oculomotor, trochlear, and abducens nerves, identified in sectioned images and surface models. Anat Rec (Hoboken). 2015; 298(2):436–443. PMID:
25212480.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download