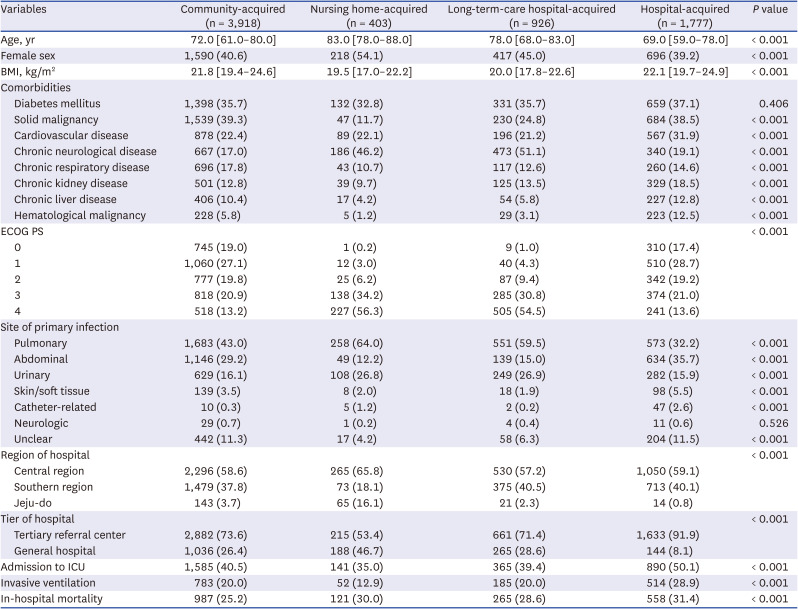
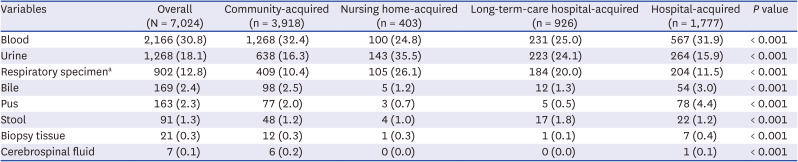
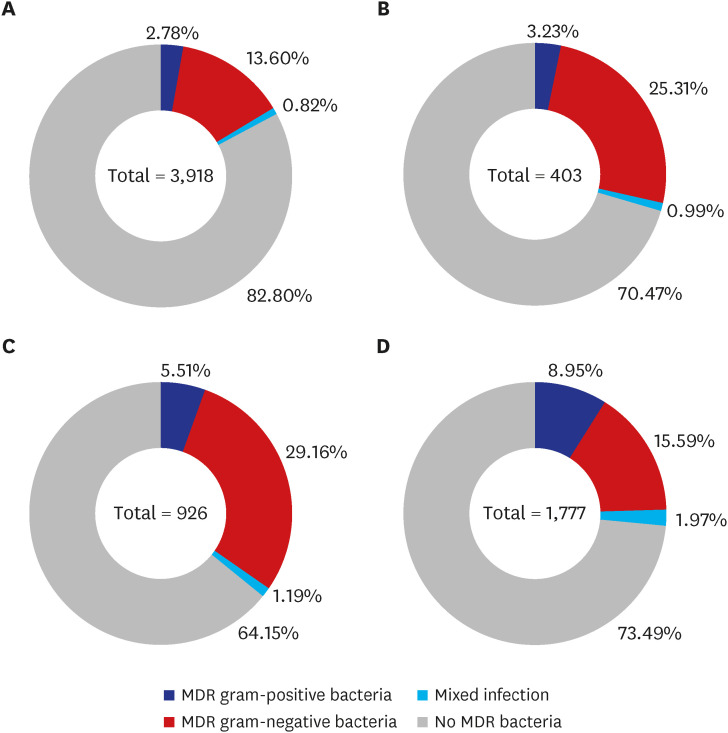
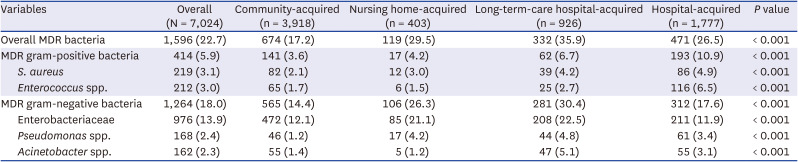
Definition of multidrug-resistant bacteria
# Staphylococcus aureus
- MRSA was considered an MDR pathogen.
- Considered as MDR pathogen if resistant to three or more categories of antibiotics below:
1. Aminoglycosides: Gentamicin
2. Ansamycins: Rifampin, rifampicin
3. Anti-MRSA cephalosporins: Ceftaroline
4. Anti-staphylococcal beta-lactams: Oxacillin, Cefoxitin
5. Fluoroquinolones: Ciprofloxacin, Levofloxacin, Moxifloxacin
6. Folate pathway inhibitors: TMP/SMX
7. Fucidanes: Fusidic acid
8. Glycopeptides: Teicoplanin, Vancomycin
9. Lincosamides: Clindamycin
10. Lipopeptides: Daptomycin
11. Macrolides: Azithromycin, Erythromycin
12. Oxazolidinones: Linezolid
13. Phenicols: Chloramphenicol
14. Phosphonic acids: Fosfomycin
15. Streptogramins: Quinupristin-dalfopristin
16. Tetracyclines: Doxycycline, Minocycline, Tetracycline
17. Glycylcyclines: Tigecycline
# Enterococcus spp.
- Considered as MDR pathogen if resistant to three or more categories of antibiotics below:
1. Aminoglycosides: Gentamicin
2. Streptomycin: Streptomycin
3. Penicillins: Ampicillin, Penicillin
4. Carbapenems: Doripenem, Imipenem, Meropenem
5. Fluoroquinolones: Ciprofloxacin, Levofloxacin, Moxifloxacin
6. Glycopeptides: Teicoplanin, Vancomycin
7. Lipopeptides: Daptomycin
8. Oxazolidinones: Linezolid
9. Streptogramins: Quinupristin-dalfopristin
10. Tetracycline: Doxycycline, Minocycline, Tetracycline
11. Glycylcyclines: Tigecycline
# Enterobacteriaceae spp.
- Considered as MDR pathogen if resistant to three or more categories of antibiotics below:
1. Aminoglycosides: Amikacin, Gentamicin, Tobramycin
2. Anti-MRSA cephalosporins: Ceftaroline
3. Penicillins: Ampicillin
4. Penicillins + beta-lactamase inhibitors: Amoxicillin/clavulanate, Ampicillin/Sulbactam
5. Antipseudomonal penicillins + beta-lactamase inhibitors: Piperacillin/tazobactam
6. 1st and 2nd generation cephalosporins: Cefazolin, Cefuroxime
7. 3rd and 4th generation cephalosporins: Cefepime, Cefotaxime, Ceftazidime, Ceftriaxone
8. Cephamycins: Cefoxitin, Cefotetan
9. Monobactams: Aztreonam
10. Carbapenems: Doripenem, Ertapenem, Imipenem, Meropenem
11. Fluoroquinolones: Ciprofloxacin, Levofloxacin, Moxifloxacin
12. Folate pathway inhibitors: TMP/SMX
13. Phenicols: Chloramphenicol
14. Phosphonic acids: Fosfomycin
15. Polymyxins: Colistin
16. Tetracyclines: Doxycycline, Minocycline, Tetracycline
17. Glycylcyclines: Tigecycline
# Pseudomonas aeruginosa
- Considered as MDR pathogen if resistant to three or more categories of antibiotics below:
1. Aminoglycosides: Amikacin, Gentamicin, Tobramycin
2. Antipseudomonal cephalosporins: Ceftazidime, Cefepime
3. Antipseudomonal penicillins + beta-lactamase inhbitors: Piperacillin/tazobactam
4. Antipseudomonal carbapenems: Doripenem, Imipenem, Meropenem
5. Antipseudomonal fluoroquinolones: Ciprofloxacin, Levofloxacin
6. Monobactams: Aztreonam
7. Phosphonic acids: Fosfomycin
8. Polymyxins: Colistin, Polymyxin B
# Acinetobacter spp.
- Considered as MDR pathogen if resistant to three or more categories of antibiotics below:
1. Aminoglycosides: Amikacin, Tobramycin, Gentamicin
2. Penicillins + beta-lactamase inhibitors: ampicillin/sulbactam
3. Antipseudomonal penicillins + beta-lactamase inhibitors: Piperacillin/tazobactam
4. Antipseudomonal carbapenems: Doripenem, Imipenem, Meropenem
5. Antipseudomonal fluoroquinolones: Ciprofloxacin, Levofloxacin
6. Extended-spectrum cephalosporins: Cefepime, Cefotaxime, Ceftazidime, Ceftriaxone
7. Folate pathway inhibitors: TMP/SMX
8. Polymyxins: Colistin, Polymyxin B
9. Tetracyclines: Doxycycline, Minocycline, Tetracycline




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download