RESULTS
Study population
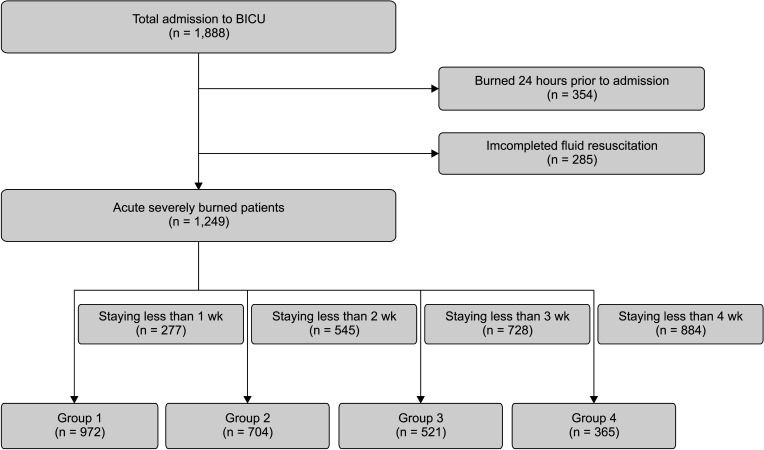
In this study, 1,888 patients admitted to the BICU between July 2012 and June 2021, within 2 days after sustaining a burn injury and receiving fluid resuscitation, were included. Three hundred fifty-four patients who suffered burn injuries within 24 hours before admission and 285 patients who did not undergo complete fluid resuscitation were excluded from the study. The remaining 1,249 eligible patients were divided into 4 groups based on their respective time points. Nine hundred seventy-two patients staying longer than a week were included in group 1,704 patients staying longer than 2 weeks in group 2,521 patients staying longer than 3 weeks in group 3, and 365 patients staying longer than 4 weeks in group 4.
Fig. 1 provides a flowchart of the enrolled patients described.
In group 1,292 patients (23.4%) died. The median of overall ages was 51 years (IQR, 41–60 years), with a male predominance of 1,016 (81.3%). The median TBSA was 31%, and inhalation injury was present in 509 (40.8%). The median length of stay in the ICU was 15 (IQR, 7–30) days. The median ABSI, rBaux, Hangang, APACHE IV, and SOFA scores were 8, 91, 133, 37, and 3, respectively. The comorbidities were hypertension in 221 (17.7%), diabetes mellitus in 106 (8.5%), hyperlipidemia in 37 (3.0%), and cardiovascular disease in 26 (2.1%) (
Table 1). The demographics for groups 1–4 are summarized in
Supplementary Tables 1–
4.
Table 1
Demographics of eligible burn patients

Prediction performance
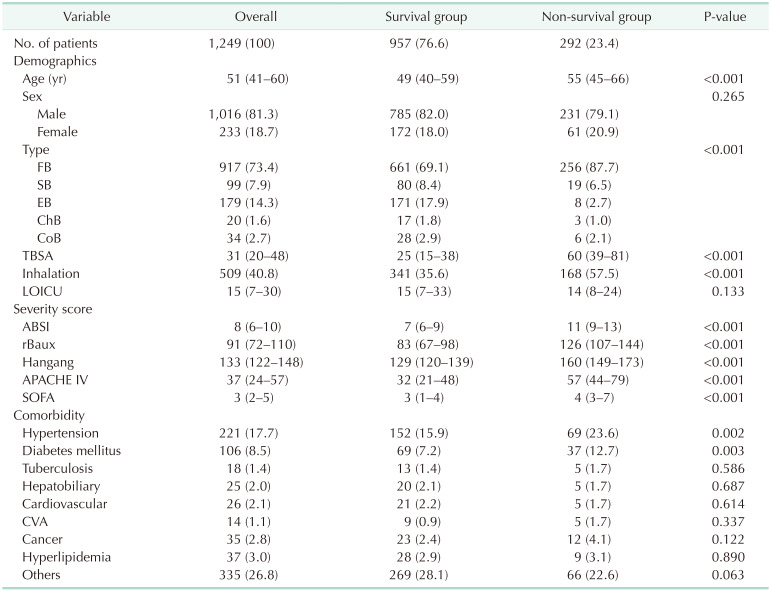
We evaluated a total of 23 biomarkers checked at least every 4 days. These were as follows: WBC count, hematocrit, platelet, RDW, neutrophil, lymphocyte, BUN, creatinine, AST, ALT, total bilirubin, direct bilirubin, albumin, glucose, CK, LD, pH, pCO2, pO2, bicarbonate, lactate, sodium, and potassium. Among them, we reported 5 biomarkers showing high-performance power. Mean Harrell’s C-index of pH during the study period was the highest with 0.816, platelets were the 2nd with 0.807, creatinine with 0.796, RDW with 0. 778, and lactate with 0.759 in order.
In Harrell’s C-index (95% confidence interval) in group 1, creatinine was 0.822 (0.794–0.850), RDW was 0.806 (0.778–0.835), and pH was 0.807 (0.778–0.835) in order (
Fig. 2). In group 2, platelet was the highest with 0.832 (0.801–0.862), pH was next with 0.824 (0.795–0.853), and creatinine was 0.811 (0.778–0.843) (
Fig. 3). In Harrell’s C-index for group 3, pH was 0.817 (0.773–0.861), platelet was 0.793 (0.750–0.835), and creatinine was 0.774 (0.723–0.825) (
Supplementary Fig. 2). In group 4, pH was the highest with 0.816 (0.757–0.874), platelet was 0.809 (0.751–0.867), and creatinine was 0.777 (0.714–0.840) in order (
Supplementary Fig. 3). The Harrell’s C-index of lactate was lower than other biomarkers, 0.794 (0.764–0.824) in group 1, 0.765 (0.726–0.804) in group 2, 0.735 (0.679–0.791) in group 3, and 0.742 (0.674–0.810) in group 4.
Fig. 2
Survival curve and Harrell’s C-index for each biomarkers in group 1. (A) Platelet, (B) red cell distribution width (RDW), (C) creatinine, (D) pH, and (E) lactate.
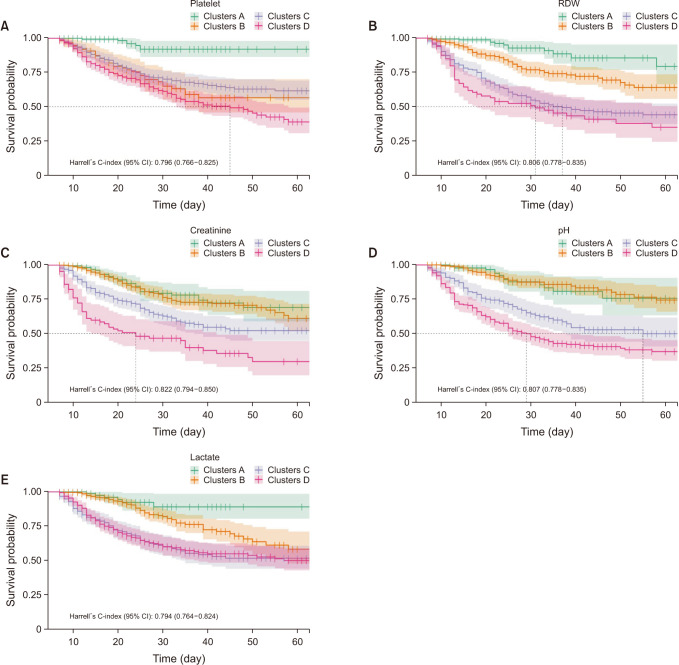

Fig. 3
Survival curve and Harrell’s C-index for each biomarkers in group 2. (A) Platelet, (B) red cell distribution width (RDW), (C) creatinine, (D) pH, and (E) lactate.

Longitudinal profile
We divided each laboratory test into 4 clusters, A through D, according to the shape pattern assigned by kmlSahpe packages using
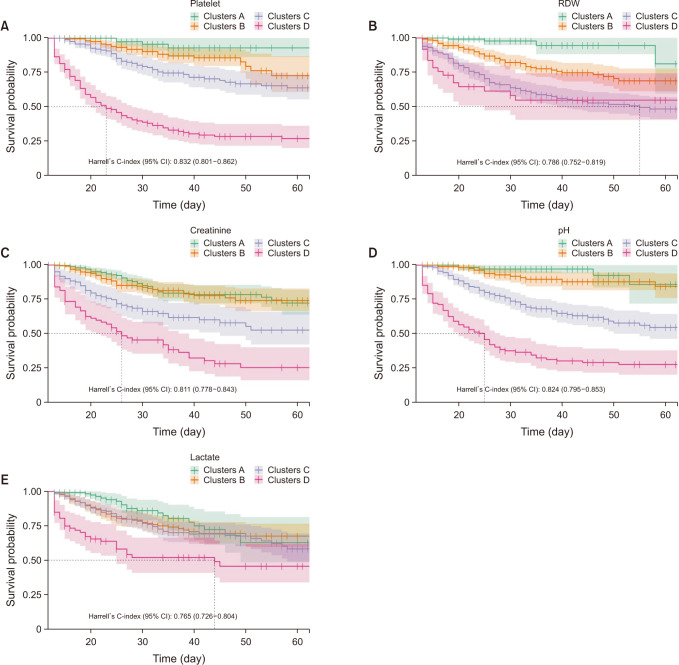
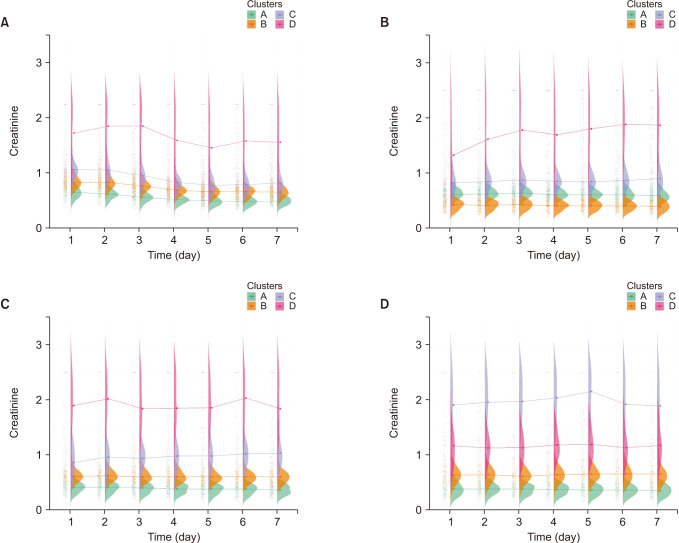
k-means clustering and rearranged according to the mortality rate of each cluster. In pH, longitudinal profiles are a bit different according to the group. For example, in group 1, clusters C and D crossed between days 2 and 3 and showed a flat pattern in groups 2, 3, and 4 (
Fig. 4). For platelets, longitudinal profiles showed different patterns between groups and even with other biomarkers. Platelets also showed a cross between days 3 and 4 in group 1, and platelet values reflect similar ebb/flow phases and showed increasing trends in group 2 (
Fig. 5). In creatinine, flat patterns were shown in all groups, with no cross line. Cluster D, with the highest RDW level, had the highest mortality rate except for group 4 (
Fig. 6). RDW also showed a cross between days 1 and 3 in group 1, and flat patterns were shown in other groups, with no cross line (
Supplementary Fig. 4). The raincloud plot for lactate showed a cross in group 1 and liner pattern were shown in other groups (
Supplementary Fig. 5). The mortality rate for all clusters of each biomarker is shown in
Supplementary Tables 5–
9.
Fig. 4
The raincloud plot of pH for each clusters. (A) Group 1, (B) group 2, (C) group 3, and (D) group 4.

Fig. 5
The raincloud plot of platelet for each clusters. (A) Group 1, (B) group 2, (C) group 3, and (D) group 4.
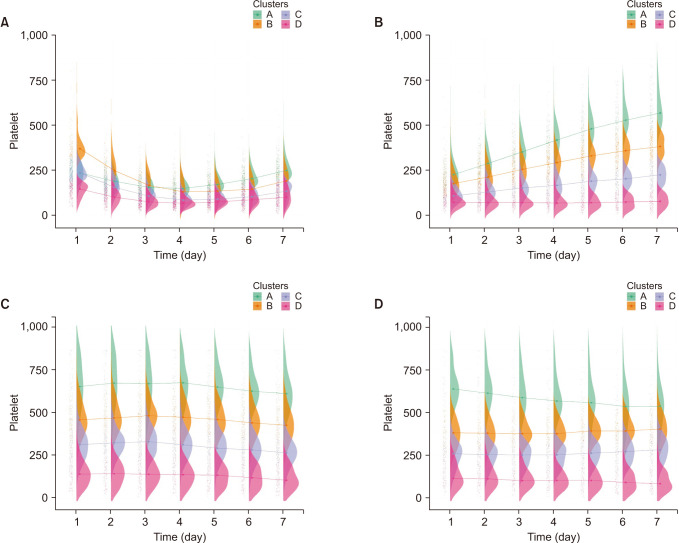

Fig. 6
The raincloud plot of creatinine for each clusters. (A) Group 1, (B) group 2, (C) group 3, and (D) group 4.

DISCUSSION
We found overall pH, platelet, lactate, and RDW showed higher performances in mortality prediction. However, they showed different longitudinal profiles. Platelets and lactate showed various changing patterns according to the condition or situation of patients, such as the ebb/flow phase. These markers seem sensitive to patient conditions. However, creatinine and RDW showed relatively consistent patterns regardless of each group. We assume that creatinine and RDW reflect hidden/unknown mechanisms more than phenotype/clinical manifestations. Physicians need to evaluate these biomarkers under the mechanism of why these phenomena were developed.
Platelets are activated with spontaneous aggregation in burns. However, the mechanism of platelet dysfunction is unclear. Regardless, this dysfunction makes a burn victim vulnerable to infection, prevents wound healing, and is associated with coagulopathy [
7]. Platelet count trajectories reported that longitudinal changes were strongly associated with mortality in burns [
8]. The mean overall C-index was as high as 0.807, a bit lower than the report that showed 0.848 at admission when combined with the rBaux score, 0.779 on day 3 combined with TBSA [
9]. However, these were combined with the rBaux score and TBSA, respectively [
9].
RDW is a predictor associated with mortality in critical illness patients and higher in non-survival burn patients group and different time trends [
10]. RDW also has the potential power of predicting morbidity, mortality of infectious disease, and sepsis [
11]. Few studies have reported on the predictive power for mortality in burn patients. In this study, the cluster with higher RDW level presented higher mortality rates, and the RDW exhibits a flat pattern rather than an ascending or descending slope pattern; this pattern was different compared to other markers. We assume that RDW is more independent of the patient's condition, whether the patient's condition is hypermetabolic or inflammatory.
Creatinine is commonly used to assess glomerular filtration and renal function in critically ill patients, and high creatinine levels are associated with increased morbidity and mortality [
12]. In this study, creatinine had a C-index of 0.796 because creatinine is known as a predictor of acute kidney injury (AKI), which is the main complication of burn injury and develops in at least 25% of patients with severe burns [
13]. Therefore, the management of AKI is one of the mainstays of burn management. The causes of AKI in burn patients are different and classified into early and late AKI according to the point of AKI development [
14]. Early AKI is attributed to fluid resuscitation volumes and the degree of burn shock. In contrast, late AKI is caused by sepsis, nephrotoxic agents, and multiorgan failure [
1516]. Therefore, biomarkers should be considered according to their mechanism. Thus, longitudinal profiles in this study could be different according to the developed mechanisms of AKI.
Lactate is known as a marker of cellular hypoxia and shock. Its correlation with mortality due to burns has been reported [
17]. Lower pH and elevated lactate levels have a strong association with mortality. In early burn stages, these markers can be attributed to local or general tissue hypoperfusion induced by burn shock or over-hydration during fluid resuscitation [
18]. These markers are also known as predictors of sepsis, which undoubtedly developed in severely burned patients. Therefore, it is necessary to look at these markers with this mechanism of disease progression in mind, and this difference leads to a change in the longitudinal profile.
To the best of our knowledge, this study is the first to use longitudinal data categorized using
k-means clustering algorithms. This means we could find heterogeneity without human selection bias using the most extensive dataset available to us. Despite being a widely used and effective algorithm for data clustering, the
k-means method has limitations. One significant limitation is determining the optimal number of clusters, which is a longstanding and complex problem in statistical analysis. There are various approaches to addressing this issue, including parametric, nonparametric, and approaches based on minimizing the number of clusters. Clinical relevance has also been proposed as a criterion for selecting the optimal number of clusters [
5]. In this study, the authors chose the optimal number of clusters to be 4 based on clinical relevance. The missing rate of missing lactate values in this study was substantial, ranging from 40% to 50%. Therefore, we employed the copyMean method for imputing the missing values; this is a widely used technique for predicting missing data in longitudinal studies. It is important to consider the high rate of missing values when interpreting the findings of this study. Additionally, as this study was conducted at a single center in 1 country, there is a potential for geographical bias that should be taken into consideration by the authors. Consequently, these findings cannot be generalized to other populations. In the future, our study design could help validate similar studies in various other institutions. Additionally, we divided into 4 groups according to time periods, which can lead to survivorship bias. However, this study assessed various biomarkers’ trajectories over time. The pathophysiology and treatment of burns evolve over time, and encompass fluid resuscitation, hypermetabolic phases, and possible complications such as AKI, sepsis, and acute respiratory distress syndrome. Therefore, the cohort was divided into 4 groups to find specific patterns of biomarkers.
We used longitudinal clinical data and unsupervised clustering algorithms to evaluate routinely collected biomarkers using k-means clustering to find insights into hidden mechanisms. Platelet, RDW, lactate, creatinine, and pH were some of the main predictors reported in other studies, but we found that they showed different longitudinal profiles. Creatinine and RDW showed even patterns, while the other markers showed different patterns according to the patients’ situations. These markers can provide clues into the underlying mechanism and help predict mortality, but the predictors that should be closely monitored may vary over time. The pH consistently demonstrated a high C-index, while creatinine and platelets displayed opposite effects. These results suggest that physicians can infer potential underlying medical conditions and focus on identifying hidden diseases. Creatinine and RDW exhibited consistent patterns, indicating that in situations where other biomarkers showed significant fluctuations, these markers may be useful for predicting mortality.






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download