Abstract
Purpose
Cytomegalovirus (CMV) infection is common in immunocompromised patients. Enterocolitis caused by CMV infection can lead to perforation and bleeding of the gastrointestinal (GI) tract, which requires emergency operation. We investigated the demographics and outcomes of patients who underwent emergency operation for CMV infection of the GI tract.
Methods
This retrospective study was conducted between January 2010 and December 2020. Patients who underwent emergency GI operation and were diagnosed with CMV infection through a pathologic examination of the surgical specimen were included. The diagnosis was confirmed using immunohistochemical staining and evaluated by experienced pathologists.
Results
A total of 27 patients who underwent operation for CMV infection were included, 18 of whom were male with a median age of 63 years. Twenty-two patients were in an immunocompromised state. Colon (37.0%) and small bowel (37.0%) were the most infected organs. CMV antigenemia testing was performed in 19 patients; 13 of whom showed positive results. The time to diagnose CMV infection from operation and time to start ganciclovir treatment were median of 9 days. The reoperation rate was 22.2% and perforation was the most common cause of reoperation. In-hospital mortality rate was 25.9%.
Conclusion
CMV infection in the GI tract causes severe effects, such as hemorrhage or perforation, in immunocompromised patients. When these outcomes are observed in immunocompromised patients, suspicion of CMV infection and further evaluation for CMV detection in tissue specimens is required for proper treatment.
Cytomegalovirus (CMV) infection of the gastrointestinal (GI) tract is one of the major causes of morbidity and mortality in immunocompromised patients through the reactivation of a latent virus or primary infection [123]. Risk factors for CMV disease include inflammatory bowel disease, use of steroids, human immunodeficiency virus (HIV) infection, ongoing chemotherapy, and history of solid organ or bone marrow transplant [456]. CMV infection of the GI tract presents with various symptoms, such as fever, abdominal pain, and diarrhea [47]. Furthermore, CMV infection of the GI tract can cause ulceration, which leads to hemorrhage or perforation; in these cases, the terminal ileum and colon are most frequently involved [8910]. Since the clinical signs of inflammation are commonly masked in immunocompromised patients, the diagnosis of perforation could be delayed and consequently lead to fatal results. CMV infection can be diagnosed using serologic or virological tests; however, the gold standard for diagnosis of tissue-invasive CMV infection is through a pathologic confirmation of the tissue using immunohistochemistry (IHC) [11121314]. Previous studies have reported CMV infection with intestinal perforation in immunocompromised patients, although most of them were case reports [151617]. Our study was conducted to evaluate the demographics and outcomes of patients who underwent operation due to CMV infection of the GI tract.
The study protocol was approved by the Institutional Review Board of Samsung Medical Center (No.2021-09-066); it was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to retrospective study.
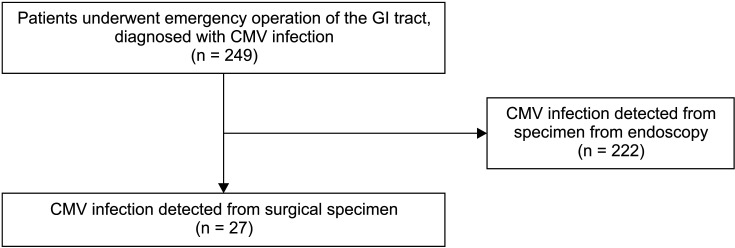
Patients (aged >18 years) who underwent emergency operation of the GI tract between January 2010 and December 2020 were retrospectively investigated. Initially, 249 patients diagnosed with CMV infection based on the pathologic findings of specimens from the GI tract were included. A total of 222 patients diagnosed with CMV infection using biopsy specimens obtained during endoscopy were excluded from this study. Finally, 27 patients were enrolled in the study (Fig. 1). Patients with abdominal pain, symptoms, and signs of intraabdominal infection underwent abdomen-pelvis CT for the diagnosis. Patients with free air in the intraabdominal cavity or suspected of perforation of the GI tract underwent emergency operation. Similarly, patients with intractable GI tract bleeding and small bowel obstruction due to strictures also underwent emergency operation. Patient demographic data and clinical data were collected using a chart review. Data on patient age, sex, height, body weight, organ involvement, operation type, underlying disease, reason for operation, perioperative estimated blood loss, laboratory results, pathologic results, time to diagnosis, time to treatment with ganciclovir, and survival were collected.
The primary outcome was reoperation after the primary operation, and the secondary outcome was in-hospital mortality due to CMV infection. CMV infection was diagnosed using immunohistochemical staining of the surgical specimen and evaluated by experienced pathologists. The results of enrolled patients were reviewed by an expert pathology specialist. Immunohistochemical stains for CMV were performed on 2-µm tissue sections. Deparaffinized slides were stained with anti-CMV (mouse monoclonal antibody, clones CCH2 and DDG9, 1:40 dilution; Dako). Slides were incubated in the Bond Epitope Retrieval Solution 2 at 100 ℃ for 20 minutes and stained on the Bond III Autostainer (Leica Microsystems). CMV infection in the blood was detected using a CMV pp65 antigenemia assay with immunofluorescence staining. CMV infection was defined as a CMV pp65 antigen-positive cell number greater than 10 positive cells per 400,000 WBCs. In cases wherein CMV infection was detected in the surgical specimen, antiviral induction therapy was initiated with intravenous ganciclovir (5 mg/kg, twice daily for 2 weeks, with renal dose adjustment if needed) [18]. If the patient was discharged before the expected treatment date, oral valganciclovir was prescribed (1 g, 3 times daily). The treatment period was extended depending on the patient’s condition.
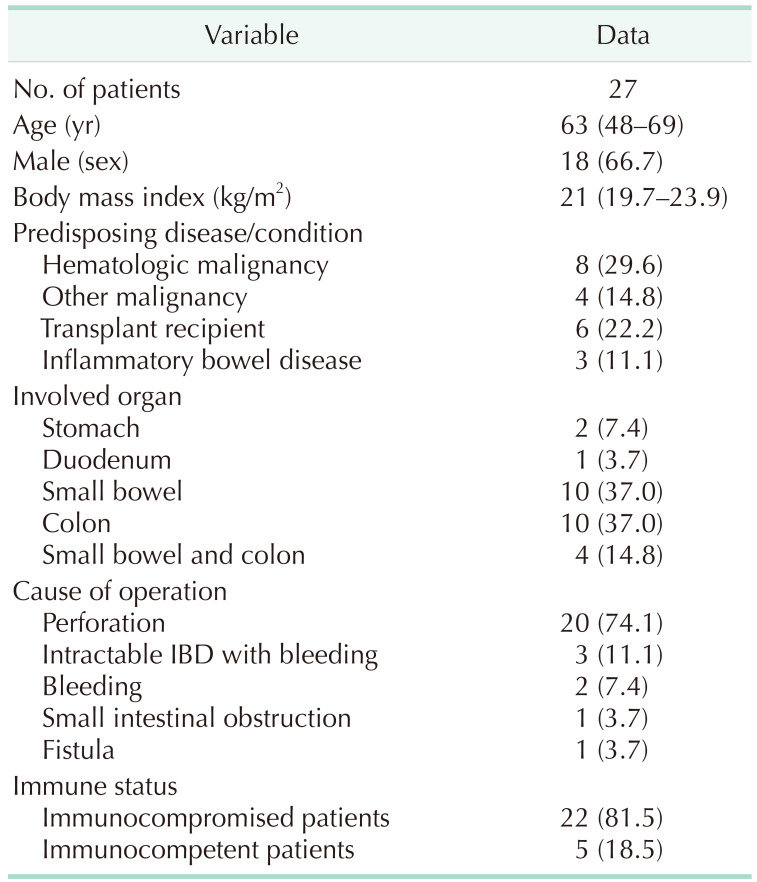
The demographics of the patients who underwent operation due to CMV infection are presented in Table 1. The median age was 63 years (interquartile range [IQR], 48–69 years) and 66.7% of patients were male. Twelve patients presented with malignancy (5 with lymphoma, 2 with acute lymphoblastic leukemia, 1 with Waldenström macroglobulinemia, 3 with colorectal cancer, and 1 with stomach cancer) as an underlying disease, 6 patients were solid organ transplant recipients, and 3 patients were diagnosed with inflammatory bowel disease. Other predisposing diseases included Churg-Strauss syndrome, stroke, and HIV infection. Colon (37.0%) and small bowel (37.0%) were the most common CMV-infected organs. In 4 patients (14.8%), both the small bowel and colon were infected with CMV. Perforation was the most common cause of operation, and 20 patients (74.1%) underwent operation due to perforation. Intractable inflammatory bowel disease with bleeding was the second most common cause of operation (3 patients, 11.1%). Other reasons for operation were bleeding, small intestinal obstruction, and fistula formation between the ileum and sigmoid colon.
Primary repairs of stomach or duodenum were done to treat perforation at stomach or duodenum. Resections of small bowel or colon were done to treat perforation or bleeding at small bowel or colon. Twenty-two patients (81.5%) were immunocompromised: 8 patients with hematologic malignancy, 6 patients with solid organ transplantation, 3 patients with inflammatory bowel disease, 2 patients with solid organ cancer undergoing chemotherapy, 1 patient with HIV infection, 1 patient with Churg-Strauss syndrome, and 1 patient with stroke with bedridden status.
Among the 29 patients, 19 underwent perioperative CMV antigenemia testing and 13 patients (68.4%) showed detectable CMV antigen levels. Twenty-two patients (81.5%) underwent antiviral treatment for CMV infection using ganciclovir. Five patients did not receive antiviral therapy; 4 (14.8%) since subtotal or total colectomy was performed and 1 (3.7%) because there was no evidence of CMV colitis. Five patients were treated with ganciclovir before operation because CMV enterocolitis was diagnosed using biopsy specimens obtained during colonoscopy or the CMV antigenemia test. The median time to CMV infection diagnosis from operation was 9 days and the median time to induction of ganciclovir treatment from operation was 9 days. The median duration of treatment with ganciclovir was 21 days (IQR, 12–21 days; range, 11–75 days) (Table 2).
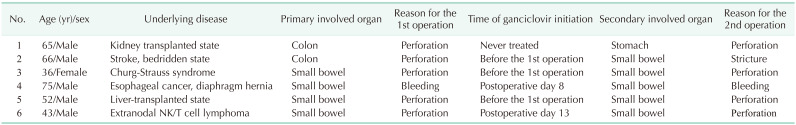
Twenty-two patients (81.5%) were admitted to the intensive care unit after operation. Ten patients (34.5%) were diagnosed with sepsis or septic shock. The median duration of hospital stay was 34 days (IQR, 21–79 days). The in-hospital mortality rate was 25.9%; of 7 patients, 4 died of septic shock, 2 died of postoperative stroke, and 1 died of respiratory failure. Six patients (22.2%) underwent reoperation (Table 3), 4 cases of which were due to perforation. Only 1 perforation site was at the previous anastomosis site. The other reasons for reoperation were small bowel stricture and small bowel bleeding. Five patients underwent antiviral treatment: 3 before the first operation and 2 after the first operation. One patient did not undergo antiviral therapy because he had undergone subtotal colectomy and was thought to have no ongoing activity. Of the 6 patients who underwent reoperation, CMV infection was detected in the operation specimen in 4 patients. One patient’s specimen did not undergo CMV infection test in reoperation, and 1 patient had negative CMV infection in specimen from reoperation.
The number of patients diagnosed with CMV infection of the GI tract and underwent operation was analyzed by year, with the median being 1.5 (IQR, 1–3). The number of patients abruptly increased from 3 in 2019 to 10 in 2020 (Fig. 2).
In this study, 22 patients (81.5%) were immunocompromised, and the colon (37.0%) and small bowel (37.0%) were the most common CMV-infected organs. Perforation was the most common cause of operation. The median time to diagnose CMV infection in specimens from operation was 8.8 days. Reoperation was performed in 22.2% of the patients. The most involved organ in reoperation was the small bowel, and the most common reason for reoperation was perforation. In-hospital mortality was 25.9%, and the most common cause of death was septic shock.
CMV infection of the GI tract is common in immunocompromised patients, such as those with HIV or malignant diseases, transplant recipients, or those undergoing chemotherapy or treatment with steroids [1920]. CMV infection is usually subclinical in immunocompetent patients but is frequently reported in previous studies; perforation has been reported to occur in these cases [212223]. CMV infection is a well-known cause of hemorrhage and ulceration in the GI tract, from the mouth to the anus, and the most common site of CMV involvement is the colon [51024]. Patra et al. [25] reported 68.5% of patients with CMV inclusion in GI mucosal biopsies were immunocompromised. Similarly, Marques et al. [4] and Le et al. [26] reported 75% of patients with upper GI CMV infection and 39.1% with CMV colitis, respectively, were immunocompromised. In our study, 81.5% were immunocompromised patients; they accounted for a high percentage of patients compared with the percentage reported in previous studies. It is thought that CMV infection in GI tract may cause more severe result like hemorrhage or perforation in immunocompromised patients. We found that the small bowel and colon were the most involved sites for CMV infection. In previous studies, the colon was reported to be the most commonly affected site in the GI tract [1227]. GI perforation due to CMV infection is a rare occurrence [9]. However, in this study, the main reason for operation was GI perforation. Therefore, close observation is needed for patients presenting with signs and symptoms of enterocolitis. Furthermore, when aggravated, a simple abdominal radiograph or abdomen-pelvis CT scan should be considered to evaluate GI tract perforation or bleeding. In our study, the in-hospital mortality rate was 25.9%, which was higher than that reported in previous studies on GI tract perforation [2829]. This implies that patients who underwent emergency operation of the GI tract due to CMV infection might have more fatal outcomes than those who underwent emergency operation owing to other reasons. Patients with CMV infection are mostly in an immunocompromised state; hence, intensive postoperative management might be needed for better outcomes.
Among total patients, 6 patients (22.2%) underwent reoperation. All 6 patients were immunocompromised state. Secondary perforation site was different from primary operation site. Reoperation rate in this study was much higher than observed in other studies of small bowel perforation due to other reasons, such as tuberculosis and Crohn disease [2830]. Also, 5 patients (83.3%) were treated with ganciclovir but 4 patients had CMV infection in specimen from second operation. This implies that the CMV-infected GI tract is vulnerable to reperforation compared to a GI tract with other etiologies of perforation, although this result could not be proven because of the small sample size. Therefore, a multicenter study should be conducted to verify this result.
The detection of CMV using hematoxylin and eosin staining of the GI tissue relies on the presence of classic CMV viral inclusions; however, viral inclusions are not commonly apparent. IHC is considered the standard for CMV detection [14], despite it not being a routine procedure for pathologic examination in many hospitals. Therefore, for highly suspected patients, IHC procedures should be considered. In our hospital, CMV study in surgical specimen was performed by clinician’s request. In this study, the number of patients abruptly increased in 2020, and 59.3% of patients were diagnosed after 2018, which was when the acute care surgery team was established in our hospital. Since then, CMV detection in surgical specimens has been emphasized; suspected patient specimens undergo further evaluation for CMV infection even after a pathologic report is created. An abrupt increase in the number of CMV-infected patients with GI tract operation in 2020 does not necessarily mean the incidence of infection increased; rather, it points to possible neglect of the diagnosis of CMV infection in previous patients. In a previous study, an early diagnosis of CMV infection reduced in-hospital mortality and increased overall survival [26]. Hence, CMV detection by a pathologist should be required when there is a high index of suspicion for infection to improve patient survival.
Although this study was the largest single-center study of CMV infection of the GI tract requiring operation regardless of the status of the patient’s immunology, the study was limited by its retrospective design. First, the CMV antigenemia test was not performed on all patients who were histologically diagnosed with CMV infection. Second, after an acute care surgery team was established in our institute, the number of requests for CMV detection in surgical specimens increased. However, since IHC for CMV antigen detection was not a routine process, it is possible that several patients with CMV infection were neglected. Third, in addition to antiviral treatment, several factors are associated with intensive care that affects survival. However, in this study, treatment during intensive care unit admission was not investigated, and the severity of disease was not considered. Finally, there was insufficient evidence to objectively evaluate the baseline immune status.
In conclusion, CMV infection of the GI tract causes severe effects, such as hemorrhage or perforation, in immunocompromised patients. Therefore, when perforation or bleeding is observed in immunocompromised patients, this causes suspicion of CMV infection, and further evaluation for CMV detection in specimens are required to evaluate the cause of perforation or hemorrhage and to treat CMV infection appropriately.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Sang Yun Ha (Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine) for review the pathologic results in our study.
References
1. Park SC, Jeen YM, Jeen YT. Approach to cytomegalovirus infections in patients with ulcerative colitis. Korean J Intern Med. 2017; 32:383–392. PMID: 28490715.
2. Ozaki T, Yamashita H, Kaneko S, Yorifuji H, Takahashi H, Ueda Y, et al. Cytomegalovirus disease of the upper gastrointestinal tract in patients with rheumatic diseases: a case series and literature review. Clin Rheumatol. 2013; 32:1683–1690. PMID: 23942768.
3. Seo M, Kim DH, Gong EJ, Ahn JY, Lee JH, Jung KW, et al. Is follow-up endoscopy necessary in upper gastrointestinal cytomegalovirus disease? Medicine (Baltimore). 2016; 95:e3389. PMID: 27175637.
4. Marques S, Carmo J, Pinto D, Bispo M, Ramos S, Chagas C. Cytomegalovirus disease of the upper gastrointestinal tract: a 10-year retrospective study. GE Port J Gastroenterol. 2017; 24:262–268. PMID: 29255766.
5. Maiorana A, Baccarini P, Foroni M, Bellini N, Giusti F. Human cytomegalovirus infection of the gastrointestinal tract in apparently immunocompetent patients. Hum Pathol. 2003; 34:1331–1336. PMID: 14691920.
6. Mohanlal RD, Karstaedt A. Cytomegalovirus infection of the gastrointestinal tract in Soweto, South Africa: A look back at the clinical and histological features over 8 years. J Infect Dis Epidemiol. 2017; 3:038.
7. Tachikawa Y, Nozawa H, Tanaka J, Nishikawa T, Tanaka T, Kiyomatsu T, et al. Colonic perforation in a patient with systemic lupus erythematosus accompanied by cytomegalovirus infection: a case report. Int J Surg Case Rep. 2016; 23:70–73. PMID: 27093690.
8. Toogood GJ, Gillespie PH, Gujral S, Warren BF, Roake JA, Gray DW, et al. Cytomegalovirus infection and colonic perforation in renal transplant patients. Transpl Int. 1996; 9:248–251. PMID: 8723195.
9. Chamberlain RS, Atkins S, Saini N, White JC. Ileal perforation caused by cytomegalovirus infection in a critically ill adult. J Clin Gastroenterol. 2000; 30:432–435. PMID: 10875475.
10. Wexner SD, Smithy WB, Trillo C, Hopkins BS, Dailey TH. Emergency colectomy for cytomegalovirus ileocolitis in patients with the acquired immune deficiency syndrome. Dis Colon Rectum. 1988; 31:755–761. PMID: 2844477.
11. O’Hara KM, Pontrelli G, Kunstel KL. An introduction to gastrointestinal tract CMV disease. JAAPA. 2017; 30:48–52.
12. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012; 14:334–342. PMID: 22588614.
13. Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004; 28:365–373. PMID: 15104299.
14. Juric-Sekhar G, Upton MP, Swanson PE, Westerhoff M. Cytomegalovirus (CMV) in gastrointestinal mucosal biopsies: should a pathologist perform CMV immunohistochemistry if the clinician requests it? Hum Pathol. 2017; 60:11–15. PMID: 27666768.
15. Goodman ZD, Boitnott JK, Yardley JH. Perforation of the colon associated with cytomegalovirus infection. Dig Dis Sci. 1979; 24:376–380. PMID: 222560.
16. Michalopoulos N, Triantafillopoulou K, Beretouli E, Laskou S, Papavramidis TS, Pliakos I, et al. Small bowel perforation due to CMV enteritis infection in an HIV-positive patient. BMC Res Notes. 2013; 6:45. PMID: 23379792.
17. Kram HB, Hino ST, Cohen RE, DeSantis SA, Shoemaker WC. Spontaneous colonic perforation secondary to cytomegalovirus in a patient with acquired immune deficiency syndrome. Crit Care Med. 1984; 12:469–471. PMID: 6325091.
18. Montoya JG, Giraldo LF, Efron B, Stinson EB, Gamberg P, Hunt S, et al. Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis. 2001; 33:629–640. PMID: 11486285.
19. Francis ND, Boylston AW, Roberts AH, Parkin JM, Pinching AJ. Cytomegalovirus infection in gastrointestinal tracts of patients infected with HIV-1 or AIDS. J Clin Pathol. 1989; 42:1055–1064. PMID: 2555397.
20. Bernard S, Germi R, Lupo J, Laverriere MH, Masse V, Morand P, et al. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: a case series. Clin Microbiol Infect. 2015; 21:1121.e1–1121.e7.
21. Cha JM, Lee JI, Choe JW, Joo KR, Jung SW, Shin HP, et al. Cytomegalovirus enteritis causing ileal perforation in an elderly immunocompetent individual. Yonsei Med J. 2010; 51:279–283. PMID: 20191024.
22. Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003; 37:1603–1606. PMID: 14689339.
23. Karakozis S, Gongora E, Caceres M, Brun E, Cook JW. Life-threatening cytomegalovirus colitis in the immunocompetent patient: report of a case and review of the literature. Dis Colon Rectum. 2001; 44:1716–1720. PMID: 11711750.
24. Nasa M, Mishra SR, Lipi L, Sud R. Common variable immunodeficiency syndrome with chronic diarrhoea. BMJ Case Rep. 2019; 12:e228240.
25. Patra S, Samal SC, Chacko A, Mathan VI, Mathan MM. Cytomegalovirus infection of the human gastrointestinal tract. J Gastroenterol Hepatol. 1999; 14:973–976. PMID: 10530492.
26. Le PH, Lin WR, Kuo CJ, Wu RC, Hsu JT, Su MY, et al. Clinical characteristics of cytomegalovirus colitis: a 15-year experience from a tertiary reference center. Ther Clin Risk Manag. 2017; 13:1585–1593. PMID: 29290686.
27. Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008; 5:47. PMID: 18371229.
28. Tan KK, Bang SL, Sim R. Surgery for small bowel perforation in an Asian population: predictors of morbidity and mortality. J Gastrointest Surg. 2010; 14:493–499. PMID: 19997984.
29. Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008; 19:577–582. PMID: 18024857.
30. Lankaram J. A study of small bowel perforations [thesis]. Madurai Medical College;2006.
Fig. 2
The number of patients who underwent emergency gastrointestinal operation due to cytomegalovirus infection by year.

Table 1
Demographics of patients who underwent operation due to cytomegalovirus infection of the gastrointestinal tract




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download