METHODS
Ethical statement
The study protocol was approved and the requirement for informed consent of individual patients was waived by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. 2022-01-152-001). This study was conducted according to the principles of the latest version (2013) of Declaration of Helsinki.
Patient selection, data collection and outcome
The participating hospital was a tertiary center that include a dedicated cancer hospital with more than 2,000 in-hospital beds (Samsung Medical Center, Seoul, Korea). From 2011 to 2021, all patients with clinically suspected PTTM (rapid RV failure with pulmonary hypertension without PTE) were derived from the pulmonary hypertension clinic registry and the electronic medical records system. Echocardiography, chest computed tomography (CT), and routine laboratory labs, including the thrombotic profile, were analyzed. Records of cancer pathology at the time of diagnosis and history of cancer treatment including surgery, radiation therapy and systemic chemotherapy were collected for primary cancer characteristics analysis. Cancer status, such as stable disease and progressive disease, was evaluated by referring to the oncologist’s records and image findings.
Clinical characteristics and courses were reviewed and analyzed with treatment strategies such as chemotherapy, anticoagulation, vasopressor administration, and intensive care such as extracorporeal membrane oxygenation (ECMO) or intubation. Since all 28 patients diagnosed with PTTM died at our hospital during treatment, the entire clinical course from diagnosis to death were verifiable with medical record.
Definition
Diagnosis of clinically suspected PTTM was made by comprehensively judging the clinical situation of high oxygen demand that was difficult to explain with lung infiltration, no evidence of PTE, signs of acute or subacute RV failure on echocardiography, and other clinical findings in advanced cancer patients. The relatively small RV cavity size compared to the increased RV systolic pressure (RVSP) and the absence of RV hypertrophy were findings suggesting acute or subacute RV failure rather than chronic RV failure. Thrombocytopenia, elevated D-dimer and combined chest CT images such as ground glass opacities (GGOs), vascular tree-in-bud sign, interstitial thickening, and mediastinal lymphadenopathy were considered as a sign of PTTM. Cases of idiopathic RV dysfunction cause by chemotherapy or main pulmonary artery metastasis were excluded.
The presence of chemo-response was defined as a case that the chemotherapy was started after the suspicion of PTTM and the patient was able to be discharged without death after chemotherapy. The criterion for decreased platelet count was defined as less than 138K/uL in women and 141K/uL in men by referring to the confidence interval of normal people. The time point at which saturation of percutaneous oxygen was checked to be less than 90% was defined as the desaturation time point. Cardiogenic shock was defined as a case in which the systolic blood pressure of less than 90 mmHg persists for more than 30 minutes or a vasopressor is required due to RV failure. Intensive care means the case of ECMO or mechanical ventilation. As for the disseminated intravascular coagulation (DIC) diagnosis, the International Society on Thrombosis and Haemostasis criteria were followed.
10) DIC was diagnosed using the following parameters: platelet count, prothrombin prolongation grade, decreased fibrinogen level, and elevated d-dimer level. When the score was 5 or greater, DIC was determined to be present. The presence of thrombocytopenia and DIC was evaluated in patients without bone metastasis.
Echocardiography
Two-dimensional echocardiography was performed using commercially available equipment. the center’s echocardiography laboratory had a standard image acquisition and analysis guideline for all transthoracic echocardiogram (TTE) examinations. Evaluation of the RV chamber size, RV function, RVSP, tricuspid annular plane systolic excursion (TAPSE), tricuspid valve (TV) S’ and inferior vena cava diameter was based on guidelines for the echocardiographic assessment of the right heart in adults.
11) The RV basal diameter was measured as the maximal short-axis dimension in the basal one third of the RV in RV–focused view. TAPSE was acquired by placing an M-mode cursor through the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole. The value of TV S’ was gained with pulsed tissue-doppler image at same area of TAPSE.
Computed tomography scanning techniques
CT scans were performed with the patient in a supine position using 64- or 128-detector row equipment. The scan coverage extended from the lung base to the level of the thoracic inlet during a single inspiratory breath-hold. All patients were administered a bolus of 90–120 mL of nonionic contrast media at a rate of 3–4 mL/second through a 18- to 20-gauge cannula into an arm or leg vein using a power injector. Immediately after administration of contrast media, a 40-mL saline bolus was injected at the same rate.
A bolus tracking technique triggered at 100 Hounsfield units on the pulmonary trunk was used before scanning in 20 patients and scanning of the other 8 patients was performed 50–55 seconds after contrast administration. The scanning parameters were as follows: 120 kVp; tube current 200 mA; beam collimation 0.6 mm; rotation time 0.4–0.5 seconds; pitch 0.9–1; and reconstructed image thickness 1.5 mm with automatic exposure control. All examinations included multi-planar reconstructions in the coronal plane.
On chest CT, the following features were evaluated by a 20-year-experienced radiologist, K.M.Y.: the RV:left ventricle (LV) ratio (RV:LV ratio), diameter of main pulmonary artery, interventricular septal flattening or bowing to LV, presence of pericardial effusion, presence of PTE, vascular tree-in-bud sign, centrilobular GGOs, peripheral GGOs, interstitial thickening suggesting lymphangitic metastasis, metastatic nodules, pleural effusion and thickening, and lymph node enlargement. To calculate the RV:LV ratio, the maximal diameters of both the right and left ventricular cavities were measured with axial CT scans.
Statistical methods
Continuous variables are presented as mean ± standard deviation or median (interquartile range) using the Student’s t-test or Mann-Whitney U test. The Shapiro-Wilk test was used for normality assumption of continuous variables. Categorical variables were compared between groups using the χ2 test or Fisher’s exact test and are presented as the numbers and relative frequencies (%). All p values were 2-sided, and p values <0.05 were considered statistically significant. Statistical analyses were performed using R statistical software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
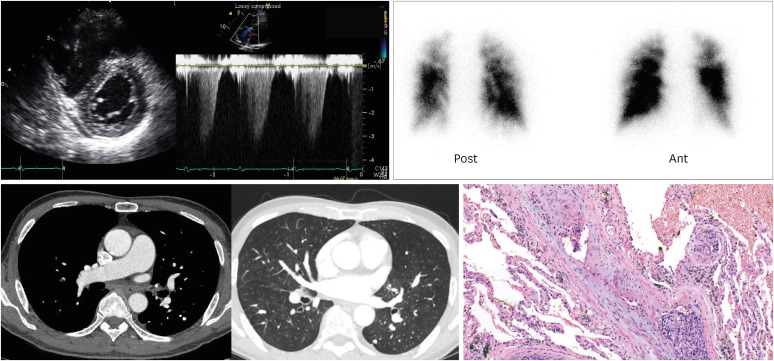
A total of 28 cases of clinically suspected PTTM, which included one biopsy-confirmed case, were included. All patients were diagnosed with cancer by biopsy, cytology, or CT images and all patients had information from echocardiography and chest CT at the time of clinically suspected PTTM. Two patients underwent lung scan and chest CT simultaneously, and the lung scan showed perfusion defects without definite evidence of pulmonary embolism in chest CT. The patient confirmed by biopsy was diagnosed with advanced gastric cancer 2 years prior and had undergone surgery after adjuvant chemotherapy. Bronchoscopy biopsy was performed due to lack of clues to explain the patient’s severe dyspnea, but the pathology was inconclusive. Therefore, video-assisted thoracoscopic surgery was proceeded, surgical biopsy specimen showed typical findings of PTTM (
Figure 1).
Figure 1
Representative figure of patient with pulmonary tumor thrombotic microangiopathy.
The mean age of patients was 54.1±11.0 years and 50% of the patients were male. All patients died within 6 months of diagnosis of PTTM. The most common cancer types were breast cancer (9/28, 32%), gastric cancer (7/28, 25%), non-small cell lung cancer (NSCLC) (2/28, 7%), and urothelial cancer (2/28, 7%). The most common tissue type was adenocarcinoma (22/26, 85%), followed by squamous cell carcinoma (2/26, 8%) and transitional cell carcinoma (2/26, 8%). In 2 patients, pathologic diagnosis was not possible due to their instability, but systemic cancer metastasis was confirmed with radiologic images. Before being diagnosed with PTTM, 22/28 (79%) of the patients had a progressive disease status. Among the 22 patients who received cancer treatment at least once, 21/22 (95%) patients were Stage 4 just before the diagnosis of PTTM and 8/22 (36%) patients were diagnosed with PTTM during secondary or higher chemotherapy (
Table 1).
Table 1
Demographics and primary cancer information in study population
|
Characteristic |
Value |
|
Demographics |
|
|
Age (years) (n=28) |
54.1±11.0 |
|
Sex (male) (n=28) |
14 (50.0) |
|
Primary cancer (n=28) |
|
|
Breast cancer |
9/28 (32) |
|
Gastric cancer |
7/28 (25) |
|
Non-small cell lung cancer |
2/28 (7) |
|
Urothelial cancer |
2/28 (7) |
|
Malignancy unknown origin |
3/28 (11) |
|
Etc.*
|
5/28 (18) |
|
Tissue type (n=26) |
|
|
Adenocarcinoma |
22/26 (85) |
|
Squamous cell carcinoma |
2/26 (8) |
|
Transitional cell carcinoma |
2/26 (8) |
|
Cancer stage at initial diagnosis (n=28) |
|
|
Stage 1 |
4/28 (14) |
|
Stage 2 |
3/28 (11) |
|
Stage 3 |
6/28 (21) |
|
Stage 4 |
15/28 (42) |
|
Cancer status before PTTM (n=28) |
|
|
Progression disease |
22/28 (79) |
|
Stable disease |
5/28 (18) |
|
No evidence of tumor recurrence |
1/28 (4) |
|
Cancer stage before PTTM (n=22†) |
|
|
Stage 1 |
1/22 (5) |
|
Stage 4 |
21/22 (95) |
|
Palliative chemotherapy before PTTM (n=22†) |
|
|
No palliative chemotherapy |
5/22 (23) |
|
1st chemotherapy |
9/22 (41) |
|
over 2nd chemotherapy |
8/22 (36) |
Dyspnea was present in all the patients (100%), and 2 of them were on mechanical ventilation support at the time of diagnosis. Thrombocytopenia was present in 16/25 (64%) and DIC was present in 14/25 (56%) at the time of suspected diagnosis of PTTM. Over the follow-up period, 22/25 (88%) patients developed thrombocytopenia. RVSP by TTE was 65.4±14.0 mmHg and RV diameter at base level was 45.2±5.6 mm. The most common types of electrocardiogram was ST-T change (62%) and sinus tachycardia (54%). Also, there was one case of atrial fibrillation and one case of atrioventricular block (
Table 2).
Table 2
Information of laboratory, echocardiography and electrocardiogram in study population

|
Characteristic |
Value |
|
Laboratory finding at admission |
|
|
Disseminated intravascular coagulation (n=25) |
14/25 (56) |
|
Thrombocytopenia (n=25) |
16/25 (64) |
|
NT-proBNP (pg/mL) (n=25) |
1,955 (1,001–4,273) |
|
D-dimer (ug/mL) (n=27) |
13.0 (5.9–23.4) |
|
Echocardiography during hospitalization |
|
|
RVSP (mmHg) (n=27) |
65.4±14.0 |
|
RV diameter (base, mm) (n=25) |
45.2±5.6 |
|
TAPSE (mm) (n=21) |
15.8±4.2 |
|
TV S’ (m/s) (n=20) |
0.116±0.039 |
|
Over moderate TR (n=27) |
5 (18.5) |
|
ECG (n=26) at admission |
|
|
Non-specific ST-T change |
16/26 (62) |
|
Sinus tachycardia |
14/26 (54) |
|
Low voltage in frontal lead |
7/26 (27) |
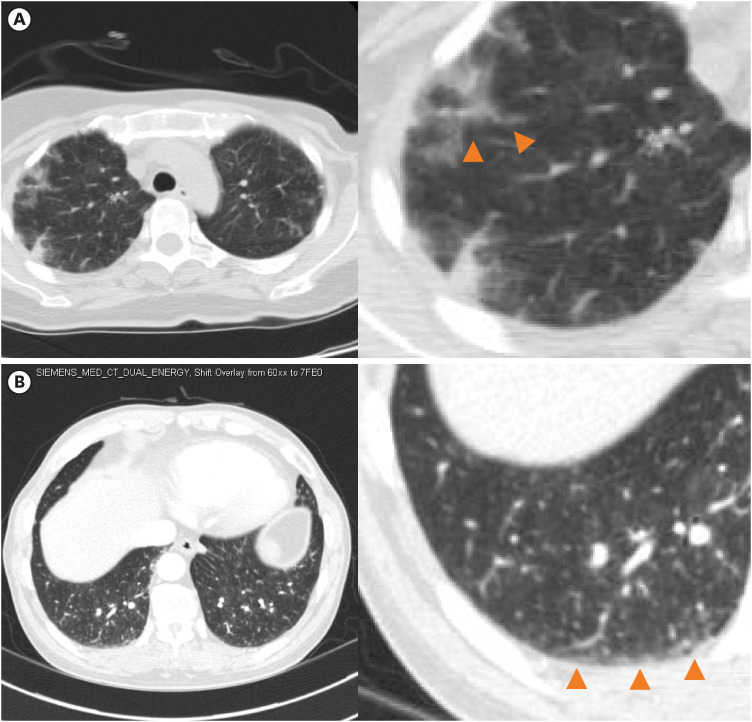
On chest CT, the median RV:LV ratio was 1.4, and the mean main pulmonary artery diameter was 3.2 cm. Interventricular flattening was observed in 75% of patients and bowing into the LV cavity was found in 11% of patients. The vascular tree-in-bud sign was found in 14%, centrilobular GGO in 46%, and peripheral wedge GGO in 32%. Interstitial thickening was confirmed in 21% and solid nodules in 32%. Pleural effusion and metastatic LNs were identified in 43% (
Table 3,
Figure 2).
Table 3
Information of chest computed tomography in study population
|
Characteristic |
Value |
|
Findings in cardiac chamber |
|
|
RV inner cavity (cm) |
3.8±0.9 |
|
LV inner cavity (cm) |
2.8 [1.9–3.2] |
|
RV:LV ratio |
1.4 [1.0–1.9] |
|
Main pulmonary artery diameter (cm) |
3.2±0.5 |
|
Interventricular septum |
|
|
|
Normal |
4/28 (14) |
|
|
Flattening |
21/28 (75) |
|
|
Bowing to LV cavity |
3/28 (11) |
|
Pericardial effusion |
|
|
|
No effusion |
14/28 (50) |
|
|
≤1 cm |
10/28 (36) |
|
|
<1 cm and ≤2cm |
4/28 (14) |
|
Findings in lung field |
|
|
Vascular tree in bud sign |
4/28 (14) |
|
Centrilobular GGOs |
|
|
|
No |
15/28 (54) |
|
|
Non diffuse or segmental |
4/28 (14) |
|
|
Diffuse |
9/28 (32) |
|
Peripheral wedge GGOs |
9/28 (32) |
|
Interstitial thickening suggesting lymphangitic meta |
6/28 (21) |
|
Solid nodules suggesting metastasis |
9/28 (32) |
|
Pleural effusion |
12/28 (43) |
|
Pleural or fissural thickening |
2/28 (7) |
|
Metastatic LNs |
12/28 (43) |
Figure 2
Representative figure of chest tomography finding in pulmonary tumor thrombotic microangiopathy. (A) Centrilobular ground glass opacities. (B) Peripheral ground glass opacities.
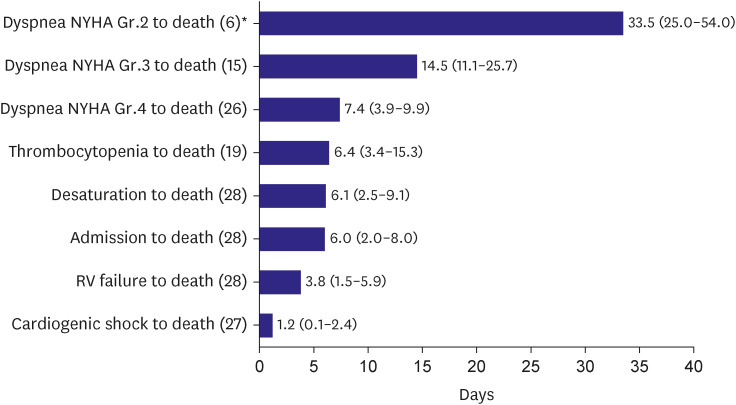
Time interval from dyspnea New York Heart Association (NYHA) Grade 2, 3, 4 to death, thrombocytopenia to death, desaturation to death, admission to death, RV failure to death, cardiogenic shock to death were 33.5 days, 14.5 days, 7.4 days, 6.4 days, 6.1 days, 6.0 days, 3.8 days and 1.2 days, respectively (
Figure 3). The more severe the grade of dyspnea, the shorter the time to death. Time to death from the presence of thrombocytopenia and desaturation was shorter than one week. Mean survival from hospital admission to death was 6 days and when there was RV failure or cardiogenic shock, survival time was less than a few days.
Figure 3
Time interval from symptoms/signs/clinical event to death. Data are presented as median (25 percentile–75 percentile).
Gr. = grade; NYHA = New York Heart Association; RV = right ventricular.
*Patients numbers used in analysis.
Each timeline was divided and compared based on the treatments given, such as chemotherapy, anticoagulation, vasopressors or inotropes, and intensive care. As a result, patients with chemotherapy lived only 7 days longer than those without chemotherapy if patients had a dyspnea NYHA Grade 4 (7.1 [3.8–8.3] days in patients without chemotherapy vs. 13.8 [6.3–54.7] days in patients with chemotherapy, p value=0.030). Of the 8 patients who received chemotherapy, 3 patients were judged to have responded to chemotherapy. Anti-coagulation did not show any differences in clinical course. In case of cardiogenic shock, inotropic treatment, and intensive care, time to death was slightly longer with statistical significance; however absolutely less than 3 days difference-which clinical meaning is doubtful. Time of cardiogenic shock to death without/with vasopressors or inotropes (0.1 [0.0–1.5] days vs. 1.9 [0.9–3.0] days, p value=0.013) and patients without/with intensive care (0.6 [0.0–1.9] days vs 2.6 [1.6–5.2] days, p value=0.006) (
Figure 4). Additionally, none of the treatments reversed the patients’ shock status.
Figure 4
Clinical time interval comparison according to treatment strategy. Data are presented as median (25 percentile–75 percentile). (A) Time interval comparison between patients with (n=8) and without chemotherapy (n=20). (B) Time interval comparison between patients with (n=16) and without anticoagulation (n=12). (C) Time interval comparison between patients with (n=14) and without vasopressor or inotrope (n=14). (D) Time interval comparison between patients with (n=9) and without intensive care (n=19).
CT = chemotherapy; Gr. = grade; NYHA = New York Heart Association; Tx = treatment.

DISCUSSION
Through this study, the researchers identified the clinical features of patients with a suspected diagnosis of PTTM and verified that chemotherapy had a disease-course-modifying-effect in those patients.
So far, studies on PTTM have been done through post-mortem data, and these have mainly focused on pathological and clinical information that has been relatively limited. Clinical information was mostly reported in the form of a case reports, making it challenging to obtain averaged data. Additionally, clinical information obtained through meta-analysis is inevitably subject to observer bias by various observers from multiple institutions. The strength of this study was that a single observer at a single institution could reduce errors caused by various observers and interpretation methods.
Moreover, the advantage of this study is that it directly compared the treatment strategy for PTTM. Anticoagulation, high-dose steroids, imatinib, drugs for pulmonary artery hypertension (PAH), and chemotherapy agents have been mentioned as possible treatments for PTTM.
12)13)14)15) However, the limitation of this treatment method was that it is based on clinical assumptions and lack statistical evidence. This paper made it possible to understand the strength and limitations of each treatment method in a validated method.
The fact that the incidence of PTTM is similar in men and women and the average age of onset is the mid-50s in this study was similar as in previous analysis with 103 cases of PTTM,
16) 30 autopsy cases,
17) and case series of severe patients.
18) As the adenocarcinoma-type tissue type was most common in this study, it was consistent with previous studies that PTTM was more common in adenocarcinoma.
6)17) Although rare, there have been cases of PTTM caused by squamous cell carcinoma in this study.
16) Most of the patients had progressive disease at the time of the PTTM diagnosis. This implies that PTTM is more likely to occur in active, progressive cancer.
In previous studies, gastric cancer was the most common primary cancer of PTTM.
6)17)19) However, in this study, gastric cancer was the second most common cause, and breast cancer the most common. This could be the result of change in the cancer epidemiology or could be a type of referral bias. In fact, the age-standardized cancer incidence rate in the 2019 Korean cancer registration statistics shows that gastric cancer incidence has decreased over the last 20 years while breast cancer incidence has increased. The common causes of PTTM that were discussed along with gastric cancer and breast cancer in previous studies were NSCLC and urothelial cancer, which were consistent with the results of this study.
17)20)
The progression of thrombocytopenia and DIC is a natural result considering the pathophysiology of PTTM. The pathophysiology of PTTM is that microscopic tumor cell emboli stimulate the coagulation cascade to cause fibrocellular intimal hyperplasia, which leads to pulmonary artery stenosis and right heart failure.
6)21)22) The DIC defined by the International Society for Heart Transplantation standard was present in 56% of cases in this study, and the result was like the previous report that DIC was present in 50%.
17)23)
In the normal population, the RV:LV ratio was less than 1 and the main pulmonary artery size is less than 2.9 cm on chest CT.
24) In this study, the median RV:LV ratio was greater than one and the mean main pulmonary artery diameter was 3.2 cm in the average age of the 50s. Also, in 86% of patients, the RV septum was abnormally located and suggestive of increased RV pressure. It was verified even in CT that most patients had pulmonary hypertension. Previous studies have mentioned that GGO, nodules, mediastinal or hilar lymphadenopathy, and septal thickening are common chest CT findings of PTTM.
17)20) A vascular-tree-in-bud pattern in chest CT identified in 14% of cases in this study is a finding that can be seen in diseases with neoplastic pulmonary emboli, such as PTTM.
25)26) In a previous study, the rate of occurrence of GGO was reported to be 82%, but the GGO type was not classified in detail.
20) In this study, GGO was divided into the centrilobular type, perifissural wedge type, and other types. All types of GGO were identified in 24 out of 28 patients (84%), which was similar to a previous study.
20) Since centrilobular GGO could be a sign of pulmonary hypertension and peripheral wedge GGO is a sign of lung parenchyma ischemia induced by decreased pulmonary artery supply,
27)28) subdividing the GGO type would be preferable. Mediastinal lymphadenopathy, which was the most common finding in a previous study (91%),
20) was confirmed in relatively few patients in the present study (43%). Interstitial thickening suggestive of lymphangitic metastasis was found in 21% of patients compared with lymphangitis carcinomatosa, which was found in 60% of patients in the previous 30 autopsy study.
17) This could mean that lymphangitic metastasis present in the pathology was not revealed on chest CT or was possibly the result of selection bias.
Due to the pathophysiology of PTTM that interferes with gas exchange, hypoxia occurred in all patients, and all complained of dyspnea.
29)30) According to past studies, it is known that it takes 4 to 12 weeks from the onset of symptoms to death,
5)16) but this can vary depending on how patients are selected or how symptoms are defined. In this study, when NYHA Grade 2 was defined as the onset of symptoms, it took about one month from the onset of symptoms to death. The strength of the present study compared to previous reports is that it quantifies the severity of symptoms.
Since most of this study population consisted of patients who received cancer therapy in this hospital, routine labs before hospitalization due to PTTM symptoms could be checked. This study found that thrombocytopenia preceded dyspnea NYHA Grade 4. If a cancer patient complaining of excessive respiratory distress with minimal pulmonary infiltration visits the hospital, checking the platelet change trend can be helpful for differential diagnosis.
The time from desaturation to death was a median of 6.1 days in this study, which is similar to the previous results that the median time was 9 days.
17) The RV failure detection to death time was 3.8 days in this study, but since echocardiography was usually performed after hospitalization, the actual time when RV failure began to progress was probably earlier than this.
This study confirmed that the survival period of about 7 days was longer in patients who received chemotherapy. In this study, 3 patients were defined as having an anticancer response, and it took 2 months, 2 months, and 5 months, respectively, from the first diagnosis of PTTM to death. In past case reports, there was also a case of survival for more than 3 to 6 months after chemotherapy.
14)15)
On the other hand, anticoagulation did not change any clinical course, which means that it is difficult to expect dramatic effects of anticoagulation in PTTM. Although vasopressor or inotrope use and intensive care significantly increased cardiogenic shock to death time, the clinical impact was insignificant because the average time difference was less than 2 days and there was no case of reversing cardiogenic shock status.
As such, chemotherapy is the only way to increase survival in PTTM, so if the patient’s condition allows, chemotherapy is recommended. If the patient’s performance status is poor for chemotherapy, it is better to avoid unnecessary treatments such as ECMO, intubation, and heparinization. And even with chemotherapy, maximal life expectancy was less than 6 months, and most cases had no response. Therefore, when hypoxia begins to appear with the diagnosis of PTTM, patients and caregivers are informed of the possibility that life expectancy is limited and to prepare for the end of life.
The first limitation of this paper is that it mainly consists of clinically suspected PTTM cases rather than biopsy confirmed cases. Due to the nature of this rapid, progressive disease, antemortem biopsy is difficult, and obtaining consent for post-mortem biopsy from the family of cancer patients is also difficult. However, it is reliable because the cases are judged by the experienced cardiologist who has treated many cancer patients over a long period of time in a tertiary hospital with a cancer center and a pulmonary hypertension expert center.
Second, since the study included cases detected by a cardiologist among patients receiving cancer therapy at a tertiary hospital, referral bias cannot be ignored. Patients who died from unknown causes or did not undergo advanced echocardiogram examination or consult a cardiologist could have been excluded from the study, leading to potential bias. And there could be a selection bias in treatment, since there was a tendency for chemotherapy to be available to patients in stable condition. Also, there is a possibility of recall bias in NYHA grading, since it depends on patient’s statement.
Third, further analysis was limited due to the small number of patients. Since only 3 patients used a drug for PAH such as bosentan or sildenafil, it was difficult to investigate the efficacy of PAH drugs. And, due to the small number of patients, no statistically significant difference could be seen in analysis between patients without chemo-response and patients with chemotherapy response.
In conclusion, rapid RV failure syndrome with a suspected diagnosis of PTTM showed a rapid progression of RV failure without PTE in advanced stages of cancer. Clinical course of this syndrome was very rapid from a few days to several weeks from symptom onset to death even with aggressive supportive therapy. Although chemotherapy could increase the time from symptom onset to death, it did not prolong the survival period for most patients, and treatments other than chemotherapy have been found to be ineffective.










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download