1. Park S, Park SJ, Park DW. Percutaneous coronary intervention for left main coronary artery disease: present status and future perspectives. JACC Asia. 2022; 2:119–138. PMID:
36339118.
2. Lee PH, Ahn JM, Chang M, et al. Left main coronary artery disease: secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol. 2016; 68:1233–1246. PMID:
27609687.
3. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994; 344:563–570. PMID:
7914958.
4. Bruschke AV, Proudfit WL, Sones FM Jr. Progress study of 590 consecutive nonsurgical cases of coronary disease followed 5-9 years. II. Ventriculographic and other correlations. Circulation. 1973; 47:1154–1163. PMID:
4709535.
5. Conley MJ, Ely RL, Kisslo J, Lee KL, McNeer JF, Rosati RA. The prognostic spectrum of left main stenosis. Circulation. 1978; 57:947–952. PMID:
639216.
6. Bangalore S, Spertus JA, Stevens SR, et al. Outcomes with intermediate left main disease: analysis from the ISCHEMIA trial. Circ Cardiovasc Interv. 2022; 15:e010925. PMID:
35411785.
7. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011; 124:2574–2609. PMID:
22064598.
8. Melly L, Torregrossa G, Lee T, Jansens JL, Puskas JD. Fifty years of coronary artery bypass grafting. J Thorac Dis. 2018; 10:1960–1967. PMID:
29707352.
9. Tan WA, Tamai H, Park SJ, et al. Long-term clinical outcomes after unprotected left main trunk percutaneous revascularization in 279 patients. Circulation. 2001; 104:1609–1614. PMID:
11581137.
10. Park DW, Park SJ. Percutaneous coronary intervention of left main disease: pre- and post-EXCEL (Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE (Nordic-Baltic-British Left Main Revascularization Study) era. Circ Cardiovasc Interv. 2017; 10:e004792. PMID:
28607000.
11. Favaloro R. Direct and indirect coronary surgery. Circulation. 1972; 46:1197–1207. PMID:
4642307.
12. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986; 314:1–6. PMID:
3484393.
13. Takaro T, Hultgren HN, Lipton MJ, Detre KM. The VA cooperative randomized study of surgery for coronary arterial occlusive disease II. Subgroup with significant left main lesions. Circulation. 1976; 54:III107–III117. PMID:
791537.
14. European Coronary Surgery Study Group. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet. 1982; 2:1173–1180. PMID:
6128492.
15. Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med. 1984; 311:1333–1339. PMID:
6333636.
16. Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988; 319:332–337. PMID:
3260659.
17. Rahimtoola SH. First percutaneous catheter intervention for left main coronary artery disease: 30 years ago. JACC Cardiovasc Interv. 2008; 1:108. PMID:
19393154.
18. O’Keefe JH Jr, Hartzler GO, Rutherford BD, et al. Left main coronary angioplasty: early and late results of 127 acute and elective procedures. Am J Cardiol. 1989; 64:144–147. PMID:
2525868.
19. Park SJ, Park SW, Hong MK, et al. Stenting of unprotected left main coronary artery stenoses: immediate and late outcomes. J Am Coll Cardiol. 1998; 31:37–42. PMID:
9426015.
20. Takagi T, Stankovic G, Finci L, et al. Results and long-term predictors of adverse clinical events after elective percutaneous interventions on unprotected left main coronary artery. Circulation. 2002; 106:698–702. PMID:
12163430.
21. Park SJ, Kim YH, Lee BK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol. 2005; 45:351–356. PMID:
15680711.
22. Pandya SB, Kim YH, Meyers SN, et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis a meta-analysis. JACC Cardiovasc Interv. 2010; 3:602–611. PMID:
20630453.
23. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012; 125:2873–2891. PMID:
22586281.
24. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012; 379:1393–1402. PMID:
22445239.
25. Buszman PE, Kiesz SR, Bochenek A, et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol. 2008; 51:538–545. PMID:
18237682.
26. Buszman PE, Buszman PP, Banasiewicz-Szkróbka I, et al. Left main stenting in comparison with surgical revascularization: 10-year outcomes of the (Left Main Coronary Artery Stenting) LE MANS trial. JACC Cardiovasc Interv. 2016; 9:318–327. PMID:
26892080.
27. Boudriot E, Thiele H, Walther T, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011; 57:538–545. PMID:
21272743.
28. Morice MC, Serruys PW, Kappetein AP, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2010; 121:2645–2653. PMID:
20530001.
29. Morice MC, Serruys PW, Kappetein AP, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014; 129:2388–2394. PMID:
24700706.
30. Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. 2019; 394:1325–1334. PMID:
31488373.
31. Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011; 364:1718–1727. PMID:
21463149.
32. Ahn JM, Roh JH, Kim YH, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J Am Coll Cardiol. 2015; 65:2198–2206. PMID:
25787197.
33. Park DW, Ahn JM, Park H, et al. Ten-year outcomes after drug-eluting stents versus coronary artery bypass grafting for left main coronary disease: extended follow-up of the PRECOMBAT trial. Circulation. 2020; 141:1437–1446. PMID:
32223567.
34. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016; 375:2223–2235. PMID:
27797291.
35. Stone GW, Kappetein AP, Sabik JF, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019; 381:1820–1830. PMID:
31562798.
36. Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016; 388:2743–2752. PMID:
27810312.
37. Holm NR, Mäkikallio T, Lindsay MM, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. 2020; 395:191–199. PMID:
31879028.
38. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016; 374:1511–1520. PMID:
27040723.
39. Ruel M, Falk V, Farkouh ME, et al. Myocardial revascularization trials. Circulation. 2018; 138:2943–2951. PMID:
30566019.
40. Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013; 62:1563–1570. PMID:
24135581.
41. Stone GW, Serruys PW, Sabik JF. PCI or CABG for left main coronary artery disease. reply. N Engl J Med. 2020; 383:292–294.
42. Parasca CA, Head SJ, Milojevic M, et al. Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX trial at 5 years. JACC Cardiovasc Interv. 2016; 9:2493–2507. PMID:
28007201.
43. Giustino G, Serruys PW, Sabik JF 3rd, et al. Mortality after repeat revascularization following PCI or CABG for left main disease: the EXCEL trial. JACC Cardiovasc Interv. 2020; 13:375–387. PMID:
31954680.
44. Giacoppo D, Colleran R, Cassese S, et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: a systematic review and meta-analysis. JAMA Cardiol. 2017; 2:1079–1088. PMID:
28903139.
45. Palmerini T, Serruys P, Kappetein AP, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017; 190:54–63. PMID:
28760214.
46. Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018; 391:939–948. PMID:
29478841.
47. Ahmad Y, Howard JP, Arnold AD, et al. Mortality after drug-eluting stents vs. coronary artery bypass grafting for left main coronary artery disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020; 41:3228–3235. PMID:
32118272.
48. Kuno T, Ueyama H, Rao SV, et al. Percutaneous coronary intervention or coronary artery bypass graft surgery for left main coronary artery disease: a meta-analysis of randomized trials. Am Heart J. 2020; 227:9–10. PMID:
32640370.
49. D’Ascenzo F, De Filippo O, Elia E, et al. Percutaneous vs. surgical revascularization for patients with unprotected left main stenosis: a meta-analysis of 5-year follow-up randomized controlled trials. Eur Heart J Qual Care Clin Outcomes. 2021; 7:476–485. PMID:
32392283.
50. Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet. 2021; 398:2247–2257. PMID:
34793745.
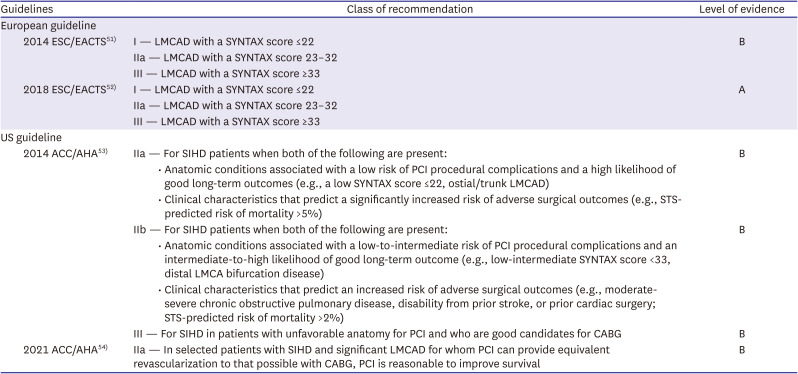
51. Authors/Task Force members. Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014; 35:2541–2619. PMID:
25173339.
52. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID:
30165437.
53. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014; 64:1929–1949. PMID:
25077860.
54. Writing Committee Members. Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 79:197–215. PMID:
34895951.
55. Kataruka A, Maynard CC, Kearney KE, et al. Temporal trends in percutaneous coronary intervention and coronary artery bypass grafting: insights from the Washington Cardiac Care Outcomes Assessment Program. J Am Heart Assoc. 2020; 9:e015317. PMID:
32456522.
56. Mohammad MA, Persson J, Buccheri S, et al. Trends in clinical practice and outcomes after percutaneous coronary intervention of unprotected left main coronary artery. J Am Heart Assoc. 2022; 11:e024040. PMID:
35350870.
57. Takahashi K, Serruys PW, Gao C, et al. Ten-year all-cause death according to completeness of revascularization in patients with three-vessel disease or left main coronary artery disease: insights from the SYNTAX Extended Survival Study. Circulation. 2021; 144:96–109. PMID:
34011163.
58. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005; 1:219–227. PMID:
19758907.
59. Naganuma T, Chieffo A, Meliga E, et al. Long-term clinical outcomes after percutaneous coronary intervention for ostial/mid-shaft lesions versus distal bifurcation lesions in unprotected left main coronary artery: the DELTA Registry (drug-eluting stent for left main coronary artery disease): a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc Interv. 2013; 6:1242–1249. PMID:
24355114.
60. Naganuma T, Chieffo A, Meliga E, et al. Long-term clinical outcomes after percutaneous coronary intervention versus coronary artery bypass grafting for ostial/midshaft lesions in unprotected left main coronary artery from the DELTA registry: a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc Interv. 2014; 7:354–361. PMID:
24630886.
61. Hyun J, Kim JH, Jeong Y, et al. Long-term outcomes after PCI or CABG for left main coronary artery disease according to lesion location. JACC Cardiovasc Interv. 2020; 13:2825–2836. PMID:
33357520.
62. Gershlick AH, Kandzari DE, Banning A, et al. Outcomes after left main percutaneous coronary intervention versus coronary artery bypass grafting according to lesion site: results from the EXCEL trial. JACC Cardiovasc Interv. 2018; 11:1224–1233. PMID:
29976358.
63. De Filippo O, Di Franco A, Boretto P, et al. Percutaneous coronary intervention versus coronary artery surgery for left main disease according to lesion site: a meta-analysis. J Thorac Cardiovasc Surg. 2021; [Epub ahead of print].
64. Arafa A, Lee HH, Eshak ES, et al. Modifiable risk factors for cardiovascular disease in Korea and Japan. Korean Circ J. 2021; 51:643–655. PMID:
34227266.
65. Silvain J, Vignalou JB, Barthélémy O, Kerneis M, Collet JP, Montalescot G. Coronary revascularization in the diabetic patient. Circulation. 2014; 130:918–922. PMID:
25199665.
66. Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012; 367:2375–2384. PMID:
23121323.
67. Lee K, Ahn JM, Yoon YH, et al. Long-term (10-year) outcomes of stenting or bypass surgery for left main coronary artery disease in patients with and without diabetes mellitus. J Am Heart Assoc. 2020; 9:e015372. PMID:
32310027.
68. Milojevic M, Serruys PW, Sabik JF 3rd, et al. Bypass surgery or stenting for left main coronary artery disease in patients with diabetes. J Am Coll Cardiol. 2019; 73:1616–1628. PMID:
30947913.
69. Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006; 114:1202–1213. PMID:
16966596.
70. Howlett JG, Stebbins A, Petrie MC, et al. CABG improves outcomes in patients with ischemic cardiomyopathy: 10-year follow-up of the STICH trial. JACC Heart Fail. 2019; 7:878–887. PMID:
31521682.
71. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022; 387:1351–1360. PMID:
36027563.
72. Wolff G, Dimitroulis D, Andreotti F, et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta-analysis. Circ Heart Fail. 2017; 10:e003255. PMID:
28087687.
73. Park S, Ahn JM, Kim TO, et al. Revascularization in patients with left main coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2020; 76:1395–1406. PMID:
32943156.
74. SCORE2-OP working group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J. 2021; 42:2455–2467. PMID:
34120185.
75. Kim KW, Kim OS. Super aging in South Korea unstoppable but mitigatable: a sub-national scale population projection for best policy planning. Spat Demogr. 2020; 8:155–173. PMID:
34222615.
76. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017; 389:1323–1335. PMID:
28236464.
77. Tran DT, Tu JV, Dupuis JY, Bader Eddeen A, Sun LY. Association of frailty and long-term survival in patients undergoing coronary artery bypass grafting. J Am Heart Assoc. 2018; 7:e009882. PMID:
30030214.
78. Park H, Ahn JM, Yoon YH, et al. Effect of age and sex on outcomes after stenting or bypass surgery in left main coronary artery disease. Am J Cardiol. 2019; 124:678–687. PMID:
31301759.
79. Ono M, Serruys PW, Hara H, et al. 10-Year follow-up after revascularization in elderly patients with complex coronary artery disease. J Am Coll Cardiol. 2021; 77:2761–2773. PMID:
34082905.
80. Senior R, Reynolds HR, Min JK, et al. Predictors of left main coronary artery disease in the ISCHEMIA trial. J Am Coll Cardiol. 2022; 79:651–661. PMID:
35177194.
81. Lindstaedt M, Spiecker M, Perings C, et al. How good are experienced interventional cardiologists at predicting the functional significance of intermediate or equivocal left main coronary artery stenoses? Int J Cardiol. 2007; 120:254–261. PMID:
17346818.
82. Hamilos M, Muller O, Cuisset T, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009; 120:1505–1512. PMID:
19786633.
83. Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014; 35:2831–2838. PMID:
24644308.
84. Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012; 5:1029–1036. PMID:
23078732.
85. Puri R, Kapadia SR, Nicholls SJ, Harvey JE, Kataoka Y, Tuzcu EM. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. JACC Cardiovasc Interv. 2012; 5:697–707. PMID:
22814774.
86. Cerrato E, Echavarria-Pinto M, D’Ascenzo F, et al. Safety of intermediate left main stenosis revascularization deferral based on fractional flow reserve and intravascular ultrasound: a systematic review and meta-regression including 908 deferred left main stenosis from 12 studies. Int J Cardiol. 2018; 271:42–48. PMID:
30223378.
87. Park H, Kang DY, Lee CW. Functional angioplasty: definitions, historical overview, and future perspectives. Korean Circ J. 2022; 52:34–46. PMID:
34989193.
88. Koo BK, Kang HJ, Youn TJ, et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005; 46:633–637. PMID:
16098427.
89. Ahn JM, Lee JY, Kang SJ, et al. Functional assessment of jailed side branches in coronary bifurcation lesions using fractional flow reserve. JACC Cardiovasc Interv. 2012; 5:155–161. PMID:
22361599.
90. Lee CH, Choi SW, Hwang J, et al. 5-Year outcomes according to FFR of left circumflex coronary artery after left main crossover stenting. JACC Cardiovasc Interv. 2019; 12:847–855. PMID:
31072505.
91. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010; 56:177–184. PMID:
20537493.
92. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017; 376:1813–1823. PMID:
28317438.
93. Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017; 376:1824–1834. PMID:
28317458.
94. Götberg M, Berntorp K, Rylance R, et al. 5-Year outcomes of PCI guided by measurement of instantaneous wave-free ratio versus fractional flow reserve. J Am Coll Cardiol. 2022; 79:965–974. PMID:
35272801.
95. Warisawa T, Cook CM, Rajkumar C, et al. Safety of revascularization deferral of left main stenosis based on instantaneous wave-free ratio evaluation. JACC Cardiovasc Interv. 2020; 13:1655–1664. PMID:
32417088.
96. Rodriguez-Leor O, de la Torre Hernandez JM, García Camarero T, et al. Instantaneous wave free ratio for the assessment of intermediate left main coronary artery stenosis: correlations with FFR/IVUS and prognostic implications: the iLITRO - EPIC07 study. Circ Cardiovasc Interv. 2022.
97. Oviedo C, Maehara A, Mintz GS, et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010; 3:105–112. PMID:
20197513.
98. Abizaid AS, Mintz GS, Abizaid A, et al. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J Am Coll Cardiol. 1999; 34:707–715. PMID:
10483951.
99. Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004; 110:2831–2836. PMID:
15492302.
100. de la Torre Hernandez JM, Hernández Hernandez F, Alfonso F, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011; 58:351–358. PMID:
21757111.
101. Park SJ, Ahn JM, Kang SJ, et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014; 7:868–874. PMID:
25147031.
102. Doi H, Maehara A, Mintz GS, et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009; 2:1269–1275. PMID:
20129555.
103. Maehara A, Mintz G, Serruys P, et al. Impact of final minimal stent area by IVUS on 3-year outcome after PCI of left main coronary artery disease: the EXCEL trial. J Am Coll Cardiol. 2017; 69:963.
104. de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014; 7:244–254. PMID:
24650399.
105. Park SJ, Kim YH, Park DW, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009; 2:167–177. PMID:
20031713.
106. Ye Y, Yang M, Zhang S, Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta-analysis. PLoS One. 2017; 12:e0179756. PMID:
28640875.
107. Kang SJ, Ahn JM, Song H, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011; 4:562–569. PMID:
22045969.
108. Ge Z, Kan J, Gao XF, et al. Comparison of intravascular ultrasound-guided with angiography-guided double kissing crush stenting for patients with complex coronary bifurcation lesions: rationale and design of a prospective, randomized, and multicenter DKCRUSH VIII trial. Am Heart J. 2021; 234:101–110. PMID:
33465369.
109. Burzotta F, Lassen JF, Lefèvre T, et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15
th consensus document from the European Bifurcation Club. EuroIntervention. 2021; 16:1307–1317. PMID:
33074152.
110. Chen SL, Zhang JJ, Han Y, et al. Double kissing crush versus provisional stenting for left main distal bifurcation lesions: DKCRUSH-V randomized trial. J Am Coll Cardiol. 2017; 70:2605–2617. PMID:
29096915.
111. Lee YJ, Suh Y, Kim JS, et al. Ticagrelor monotherapy after 3-month dual antiplatelet therapy in acute coronary syndrome by high bleeding risk: the subanalysis from the TICO trial. Korean Circ J. 2022; 52:324–337. PMID:
35129317.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download