1. Hoffman JI. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr Cardiol. 1995; 16:103–113. PMID:
7617503.
2. Lillehei CW, Cohen M, Warden HE, Ziegler NR, Varco RL. The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation. Surg Gynecol Obstet. 1955; 101:446–466. PMID:
13256320.
3. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, et al. Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22-34 years. Eur Heart J. 2004; 25:1057–1062. PMID:
15191777.
4. Nygren A, Sunnegårdh J, Berggren H. Preoperative evaluation and surgery in isolated ventricular septal defects: a 21 year perspective. Heart. 2000; 83:198–204. PMID:
10648497.
5. Monro JL, Alexiou C, Salmon AP, Keeton BR. Reoperations and survival after primary repair of congenital heart defects in children. J Thorac Cardiovasc Surg. 2003; 126:511–520. PMID:
12928652.
6. Hobbins SM, Izukawa T, Radford DJ, Williams WG, Trusler GA. Conduction disturbances after surgical correction of ventricular septal defect by the atrial approach. Br Heart J. 1979; 41:289–293. PMID:
426978.
7. Bol-Raap G, Weerheim J, Kappetein AP, Witsenburg M, Bogers AJ. Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg. 2003; 24:511–515. PMID:
14500067.
8. Visconti KJ, Bichell DP, Jonas RA, Newburger JW, Bellinger DC. Developmental outcome after surgical versus interventional closure of secundum atrial septal defect in children. Circulation. 1999; 100:II145–II150. PMID:
10567294.
9. Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation. 1988; 78:361–368. PMID:
3396173.
10. Rigby ML, Redington AN. Primary transcatheter umbrella closure of perimembranous ventricular septal defect. Br Heart J. 1994; 72:368–371. PMID:
7833197.
11. Kalra GS, Verma PK, Dhall A, Singh S, Arora R. Transcatheter device closure of ventricular septal defects: immediate results and intermediate-term follow-up. Am Heart J. 1999; 138:339–344. PMID:
10426849.
12. Kalra GS, Verma PK, Singh S, Arora R. Transcatheter closure of ventricular septal defect using detachable steel coil. Heart. 1999; 82:395–396. PMID:
10455098.
13. Sideris EB, Walsh KP, Haddad JL, Chen CR, Ren SG, Kulkarni H. Occlusion of congenital ventricular septal defects by the buttoned device. “Buttoned device” Clinical Trials International Register. Heart. 1997; 77:276–279. PMID:
9093050.
14. Knauth AL, Lock JE, Perry SB, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004; 110:501–507. PMID:
15262841.
15. Amin Z, Gu X, Berry JM, et al. New device for closure of muscular ventricular septal defects in a canine model. Circulation. 1999; 100:320–328. PMID:
10411859.
16. Yang L, Tai BC, Khin LW, Quek SC. A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). J Interv Cardiol. 2014; 27:260–272. PMID:
24773223.
17. Giugno L, Faccini A, Carminati M. Percutaneous pulmonary valve implantation. Korean Circ J. 2020; 50:302–316. PMID:
32157831.
18. Lee OH, Kim JS. Percutaneous patent foramen ovale closure after stroke. Korean Circ J. 2022; 52:801–807. PMID:
36347516.
19. Huang Y, Yan X, Lu L, et al. Transcatheter closure of doubly committed subarterial ventricular septal defects with the Amplatzer ductal occluder: initial experience. Cardiol Young. 2019; 29:570–575. PMID:
30968785.
20. Tang C, Zhou K, Shao S, et al. Transfemoral occlusion of doubly committed subarterial ventricular septal defect using the Amplatzer duct occluder-II in children. Front Cardiovasc Med. 2022; 9:837847. PMID:
35498007.
21. Zhou S, Zhao L, Fan T, et al. Perventricular device closure of doubly committed sub-arterial ventricular septal defects via a left infra-axillary approach. J Card Surg. 2017; 32:382–386. PMID:
28543756.
22. Morray BH. Ventricular septal defect closure devices, techniques, and outcomes. Interv Cardiol Clin. 2019; 8:1–10. PMID:
30449417.
23. Backer CL, Winters RC, Zales VR, et al. Restrictive ventricular septal defect: how small is too small to close? Ann Thorac Surg. 1993; 56:1014–1018. PMID:
8239793.
24. Neumayer U, Stone S, Somerville J. Small ventricular septal defects in adults. Eur Heart J. 1998; 19:1573–1582. PMID:
9820997.
25. Wongwaitaweewong K, Promphan W, Roymanee S, Prachasilchai P. Effect of transcatheter closure by Amplatzer
™ duct occluder II in patients with small ventricular septal defect. Cardiovasc Interv Ther. 2021; 36:375–383. PMID:
32462466.
26. Pillai AA, Rangasamy S, Balasubramonian VR. Transcatheter closure of moderate to large perimembranous ventricular septal defects in children weighing 10 kilograms or less. World J Pediatr Congenit Heart Surg. 2019; 10:278–285. PMID:
31084308.
27. Ghosh S, Mukherji A, Chattopadhyay A. Percutaneous closure of moderate to large perimembranous ventricular septal defect in small children using left ventricular mid-cavity approach. Indian Heart J. 2020; 72:570–575. PMID:
33357647.
28. Pedra CA, Pedra SR, Chaccur P, et al. Perventricular device closure of congenital muscular ventricular septal defects. Expert Rev Cardiovasc Ther. 2010; 8:663–674. PMID:
20450300.
29. Haddad RN, Gaudin R, Bonnet D, Malekzadeh-Milani S. Hybrid perventricular muscular ventricular septal defect closure using the new multi-functional occluder. Cardiol Young. 2020; 30:1517–1520. PMID:
32787993.
30. Kang SL, Tometzki A, Caputo M, Morgan G, Parry A, Martin R. Longer-term outcome of perventricular device closure of muscular ventricular septal defects in children. Catheter Cardiovasc Interv. 2015; 85:998–1005. PMID:
25573696.
31. Zuo J, Xie J, Yi W, et al. Results of transcatheter closure of perimembranous ventricular septal defect. Am J Cardiol. 2010; 106:1034–1037. PMID:
20854970.
32. Holzer R, Balzer D, Cao QL, Lock K, Hijazi ZM. Amplatzer Muscular Ventricular Septal Defect Investigators. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004; 43:1257–1263. PMID:
15063439.
33. Koneti NR, Sreeram N, Penumatsa RR, Arramraj SK, Karunakar V, Trieschmann U. Transcatheter retrograde closure of perimembranous ventricular septal defects in children with the Amplatzer duct occluder II device. J Am Coll Cardiol. 2012; 60:2421–2422. PMID:
23141497.
34. Muthusamy K. Retrograde closure of perimembranous ventricular septal defect using muscular ventricular septal occluder: a single-center experience of a novel technique. Pediatr Cardiol. 2015; 36:106–110. PMID:
25139246.
35. Asou T. Surgical management of muscular trabecular ventricular septal defects. Gen Thorac Cardiovasc Surg. 2011; 59:723–729. PMID:
22083689.
36. Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2013; 61:578–607. PMID:
23990117.
37. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Masuda M, Kuwano H, et al. Thoracic and cardiovascular surgery in Japan during 2012: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2014; 62:734–764. PMID:
25355583.
38. Thanopoulos BD, Rigby ML. Outcome of transcatheter closure of muscular ventricular septal defects with the Amplatzer ventricular septal defect occluder. Heart. 2005; 91:513–516. PMID:
15772216.
39. Hijazi ZM, Hakim F, Al-Fadley F, Abdelhamid J, Cao QL. Transcatheter closure of single muscular ventricular septal defects using the Amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv. 2000; 49:167–172. PMID:
10642766.
40. Chessa M, Carminati M, Cao QL, et al. Transcatheter closure of congenital and acquired muscular ventricular septal defects using the Amplatzer device. J Invasive Cardiol. 2002; 14:322–327. PMID:
12042624.
41. Arora R, Trehan V, Thakur AK, Mehta V, Sengupta PP, Nigam M. Transcatheter closure of congenital muscular ventricular septal defect. J Interv Cardiol. 2004; 17:109–115. PMID:
15104774.
42. Koneti NR, Verma S, Bakhru S, et al. Transcatheter trans-septal antegrade closure of muscular ventricular septal defects in young children. Catheter Cardiovasc Interv. 2013; 82:E500–E506. PMID:
23704080.
43. Zartner P, Christians C, Stelter JC, Hraška V, Schneider MB. Transvascular closure of single and multiple muscular ventricular septal defects in neonates and infants < 20 kg. Catheter Cardiovasc Interv. 2014; 83:564–570. PMID:
23996896.
44. Diab KA, Cao QL, Mora BN, Hijazi ZM. Device closure of muscular ventricular septal defects in infants less than one year of age using the Amplatzer devices: feasibility and outcome. Catheter Cardiovasc Interv. 2007; 70:90–97. PMID:
17585388.
45. Butera G, Carminati M, Chessa M, et al. Percutaneous closure of ventricular septal defects in children aged <12: early and mid-term results. Eur Heart J. 2006; 27:2889–2895. PMID:
17053007.
46. Amin Z, Berry JM, Foker JE, Rocchini AP, Bass JL. Intraoperative closure of muscular ventricular septal defect in a canine model and application of the technique in a baby. J Thorac Cardiovasc Surg. 1998; 115:1374–1376. PMID:
9628681.
47. Bacha EA, Cao QL, Starr JP, Waight D, Ebeid MR, Hijazi ZM. Perventricular device closure of muscular ventricular septal defects on the beating heart: technique and results. J Thorac Cardiovasc Surg. 2003; 126:1718–1723. PMID:
14688678.
48. Bacha EA, Cao QL, Galantowicz ME, et al. Multicenter experience with perventricular device closure of muscular ventricular septal defects. Pediatr Cardiol. 2005; 26:169–175. PMID:
15868323.
49. Crossland DS, Wilkinson JL, Cochrane AD, d’Udekem Y, Brizard CP, Lane GK. Initial results of primary device closure of large muscular ventricular septal defects in early infancy using perventricular access. Catheter Cardiovasc Interv. 2008; 72:386–391. PMID:
18727115.
50. Amin Z, Cao QL, Hijazi ZM. Closure of muscular ventricular septal defects: transcatheter and hybrid techniques. Catheter Cardiovasc Interv. 2008; 72:102–111. PMID:
18546234.
51. Kim SJ, Huh J, Song JY, Yang JH, Jun TG, Kang IS. The hybrid perventricular closure of apical muscular ventricular septal defect with Amplatzer duct occluder. Korean J Pediatr. 2013; 56:176–181. PMID:
23646056.
52. Kirklin JK, Castaneda AR, Keane JF, Fellows KE, Norwood WI. Surgical management of multiple ventricular septal defects. J Thorac Cardiovasc Surg. 1980; 80:485–493. PMID:
7421283.
53. Fu YC, Bass J, Amin Z, et al. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006; 47:319–325. PMID:
16412854.
54. Hijazi ZM, Hakim F, Haweleh AA, et al. Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv. 2002; 56:508–515. PMID:
12124963.
55. Bass JL, Kalra GS, Arora R, et al. Initial human experience with the Amplatzer perimembranous ventricular septal occluder device. Catheter Cardiovasc Interv. 2003; 58:238–245. PMID:
12552550.
56. Butera G, Carminati M, Chessa M, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. 2007; 50:1189–1195. PMID:
17868812.
57. Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007; 28:2361–2368. PMID:
17684082.
58. Holzer R, de Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006; 68:620–628. PMID:
16969878.
59. Predescu D, Chaturvedi RR, Friedberg MK, Benson LN, Ozawa A, Lee KJ. Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg. 2008; 136:1223–1228. PMID:
19026807.
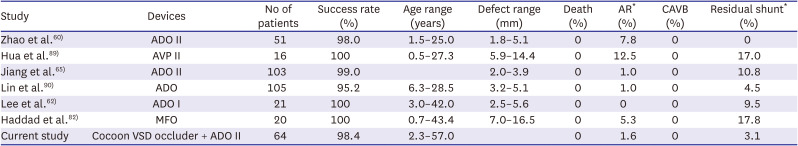
60. Zhao LJ, Han B, Zhang JJ, Yi YC, Jiang DD, Lyu JL. Transcatheter closure of congenital perimembranous ventricular septal defect using the Amplatzer duct occluder 2. Cardiol Young. 2018; 28:447–453. PMID:
29233213.
61. El-Sisi A, Sobhy R, Jaccoub V, Hamza H. Perimembranous ventricular septal defect device closure: choosing between Amplatzer duct occluder I and II. Pediatr Cardiol. 2017; 38:596–602. PMID:
28251252.
62. Lee SM, Song JY, Choi JY, et al. Transcatheter closure of perimembranous ventricular septal defect using Amplatzer ductal occluder. Catheter Cardiovasc Interv. 2013; 82:1141–1146. PMID:
23554093.
63. El Said HG, Bratincsak A, Gordon BM, Moore JW. Closure of perimembranous ventricular septal defects with aneurysmal tissue using the Amplazter duct occluder I: lessons learned and medium term follow up. Catheter Cardiovasc Interv. 2012; 80:895–903. PMID:
22907908.
64. Mijangos-Vázquez R, El-Sisi A, Sandoval Jones JP, et al. Transcatheter closure of perimembranous ventricular septal defects using different generations of Amplatzer devices: multicenter experience. J Interv Cardiol. 2020; 2020:8948249. PMID:
32161516.
65. Jiang D, Zhang J, Fan Y, et al. The efficacy and medium to long-term follow-up of transcatheter retrograde closure of perimembranous ventricular septal defects via the femoral artery with Amplatzer duct occluder II in children. Front Pediatr. 2021; 9:571407. PMID:
34113582.
66. Velasco-Sanchez D, Tzikas A, Ibrahim R, Miró J. Transcatheter closure of perimembranous ventricular septal defects: initial human experience with the Amplatzer
® membranous VSD occluder 2. Catheter Cardiovasc Interv. 2013; 82:474–479. PMID:
22431366.
67. Mallula K, Patel N, Amin Z. New design of the Amplatzer membranous VSD occluder: a step forward? Pediatr Cardiol. 2013; 34:2068–2072. PMID:
23377383.
68. Tzikas A, Ibrahim R, Velasco-Sanchez D, et al. Transcatheter closure of perimembranous ventricular septal defect with the Amplatzer
® membranous VSD occluder 2: initial world experience and one-year follow-up. Catheter Cardiovasc Interv. 2014; 83:571–580. PMID:
23703890.
69. Pedra CA, Pedra SR, Esteves CA, et al. Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv. 2004; 61:403–410. PMID:
14988905.
70. Yang J, Yang L, Wan Y, et al. Transcatheter device closure of perimembranous ventricular septal defects: mid-term outcomes. Eur Heart J. 2010; 31:2238–2245. PMID:
20801925.
71. Wang L, Cao S, Li J, et al. Transcatheter closure of congenital perimembranous ventricular septal defect in children using symmetric occluders: an 8-year multiinstitutional experience. Ann Thorac Surg. 2012; 94:592–598. PMID:
22626754.
72. Tucker EM, Pyles LA, Bass JL, Moller JH. Permanent pacemaker for atrioventricular conduction block after operative repair of perimembranous ventricular septal defect. J Am Coll Cardiol. 2007; 50:1196–1200. PMID:
17868813.
73. Yip WC, Zimmerman F, Hijazi ZM. Heart block and empirical therapy after transcatheter closure of perimembranous ventricular septal defect. Catheter Cardiovasc Interv. 2005; 66:436–441. PMID:
16216027.
74. Walsh MA, Bialkowski J, Szkutnik M, Pawelec-Wojtalik M, Bobkowski W, Walsh KP. Atrioventricular block after transcatheter closure of perimembranous ventricular septal defects. Heart. 2006; 92:1295–1297. PMID:
16449504.
75. Butera G, Gaio G, Carminati M. Is steroid therapy enough to reverse complete atrioventricular block after percutaneous perimembranous ventricular septal defect closure? J Cardiovasc Med (Hagerstown). 2009; 10:412–414. PMID:
19262403.
76. Yang R, Kong XQ, Sheng YH, et al. Risk factors and outcomes of post-procedure heart blocks after transcatheter device closure of perimembranous ventricular septal defect. JACC Cardiovasc Interv. 2012; 5:422–427. PMID:
22516400.
77. Leong MC, Alwi M. Complete atrio-ventricular heart block, a not to be forgotten complication in transcatheter closure of perimembranous ventricular septal defect - a case report and review of literature. Cardiol Young. 2021; 31:2031–2034. PMID:
34053471.
78. Li X, Li L, Wang X, Zhao HB, Zhang SY. Clinical analysis of transcatheter closure of perimembranous ventricular septal defects with occluders made in China. Chin Med J (Engl). 2011; 124:2117–2122. PMID:
21933612.
79. Zhou D, Pan W, Guan L, Ge J. Transcatheter closure of perimembranous and intracristal ventricular septal defects with the SHSMA occluder. Catheter Cardiovasc Interv. 2012; 79:666–674. PMID:
22109986.
80. Li P, Zhao XX, Zheng X, Qin YW. Arrhythmias after transcatheter closure of perimembranous ventricular septal defects with a modified double-disk occluder: early and long-term results. Heart Vessels. 2012; 27:405–410. PMID:
21643813.
81. Esteves CA, Solarewicz LA, Cassar R, Neves JR, Esteves V, Arrieta R. Occlusion of the perimembranous ventricular septal defect using CERA
® devices. Catheter Cardiovasc Interv. 2012; 80:182–187. PMID:
22431503.
82. Haddad RN, Daou LS, Saliba ZS. Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: midterm outcomes of the first middle-eastern experience. Catheter Cardiovasc Interv. 2020; 96:E295–E302. PMID:
31886940.
83. Sadiq M, Qureshi AU, Younas M, Arshad S, Hyder SN. Percutaneous closure of ventricular septal defect using LifeTech™ Konar-MF VSD occluder: initial and short-term multi-institutional results. Cardiol Young. 2022; 32:755–761. PMID:
34318740.
84. Bjorkman KR, Aldoss O, Maldonado JR, McLennan D. Transcatheter utilisation of Lifetech multifunction™ occluder device for closure of perimembranous and muscular ventricular septal defects: first use in North America. Cardiol Young. 2021; 31:1525–1527. PMID:
33766174.
85. Zhao LJ, Han B, Zhang JJ, Yi YC, Jiang DD, Lyu JL. Postprocedural outcomes and risk factors for arrhythmias following transcatheter closure of congenital perimembranous ventricular septal defect: a single-center retrospective study. Chin Med J (Engl). 2017; 130:516–521. PMID:
28229981.
86. Landman G, Kipps A, Moore P, Teitel D, Meadows J. Outcomes of a modified approach to transcatheter closure of perimembranous ventricular septal defects. Catheter Cardiovasc Interv. 2013; 82:143–149. PMID:
23225758.
87. Li H, Shi Y, Zhang S, et al. Short- and medium-term follow-up of transcatheter closure of perimembranous ventricular septal defects. BMC Cardiovasc Disord. 2019; 19:222. PMID:
31619172.
88. Saurav A, Kaushik M, Mahesh Alla V, et al. Comparison of percutaneous device closure versus surgical closure of peri-membranous ventricular septal defects: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2015; 86:1048–1056. PMID:
26257085.
89. Hua N, Aquino P, Owada CY. Transcatheter closure of perimembranous ventricular septal defects with the Amplatzer Vascular Plug-II. Cardiol Young. 2016; 26:1194–1201. PMID:
26498904.
90. Lin MT, Chen CA, Hsu JY, et al. Transcatheter closure of perimembranous ventricular septal defects with Amplatzer duct occluders. JACC Cardiovasc Interv. 2017; 10:2227–2228. PMID:
29122136.
91. Walsh MA, Coleman DM, Oslizlok P, Walsh KP. Percutaneous closure of postoperative ventricular septal defects with the Amplatzer device. Catheter Cardiovasc Interv. 2006; 67:445–451. PMID:
16489568.
92. Kouakou NY, Song J, Huh J, Kang IS. The experience of transcatheter closure of postoperative ventricular septal defect after total correction. J Cardiothorac Surg. 2019; 14:104. PMID:
31186037.
93. Ryu IH, Kim WH, Ryu AJ, et al. Percutaneous closure of an iatrogenic ventricular septal defect following concomitant septal myectomy at the time of aortic valve replacement. Korean Circ J. 2014; 44:45–48. PMID:
24497890.
94. Haas NA, Kock L, Bertram H, et al. Interventional VSD-closure with the Nit-Occlud
® Lê VSD-coil in 110 patients: early and midterm results of the EUREVECO-Registry. Pediatr Cardiol. 2017; 38:215–227. PMID:
27847970.
95. Atik-Ugan S, Saltik IL. Transcatheter closure of ventricular septal defect with Occlutech Duct Occluder. Cardiol Young. 2018; 28:598–601. PMID:
29513202.
96. Park H, Song J, Kim ES, Huh J, Kang IS. Erratum: early experiences using Cocoon occluders for closure of a ventricular septal defect. J Cardiovasc Imaging. 2018; 26:256–257. PMID:
30607396.




 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download